Antidepressant prescriptions continue to steadily grow in England
Pharmaceutical Technology
JULY 7, 2023
As quarterly antidepressant prescriptions hit 22 million, the NHS looks to cut down patient reliance.

Pharmaceutical Technology
JULY 7, 2023
As quarterly antidepressant prescriptions hit 22 million, the NHS looks to cut down patient reliance.

Fierce Pharma
JULY 7, 2023
As demand skyrockets for GLP-1 drugs that can trigger significant weight loss, unauthorized versions of the treatments have started to fill pharmacies. | In late May, the FDA warned of illegal knockoffs of Novo Nordisk’s Ozempic and Wegovy. And now, the Danish company has filed its second wave of lawsuits against pharmacies in the U.S. that are producing the copycats.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 7, 2023
AC-0676 is under clinical development by Accutar Biotechnology and currently in Phase I for Follicular Lymphoma.

XTalks
JULY 7, 2023
A recent study in JAMA Network Open analyzed the differences in commercial negotiated prices and cash prices between physician-owned hospitals (POHs) and non-POHs. Data on commercial negotiated prices and cash prices for eight specific services were analyzed from 156 POHs and 1,116 non-POHs in 78 hospital referral regions (HRRs). The eight services included spinal injection, therapeutic physical therapy exercise, magnetic resonance imaging (MRI) scan of the lower spinal canal, computed tomograph

Pharmaceutical Technology
JULY 7, 2023
Thermo Fisher Scientific has signed a definitive agreement for the acquisition of CorEvitas from Audax Private Equity for $912.5m in cash.

XTalks
JULY 7, 2023
Pfizer’s alopecia JAK inhibitor Litfulo (ritlecitinib) has become its fourth US Food and Drug Administration (FDA) approved product in the past several weeks. Litfulo is entering the alopecia market to rival Eli Lilly’s Olumiant (baricitinib), which was approved as the first drug for alopecia areata a year ago. While Olumiant only has approval for adults, Litfulo has a slight edge as it is indicated for both adults and adolescents 12 years of age and older — it’s the first drug approved for trea
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

BioPharma Reporter
JULY 7, 2023
The first and only approved anti-amyloid Alzheimer's Disease (AD) treatment shown to reduce the rate of disease progression and slow cognitive impairment has been granted approval by the FDA.

Pharmaceutical Technology
JULY 7, 2023
AC-0676 is under clinical development by Accutar Biotechnology and currently in Phase I for Follicular Lymphoma.

Pharma Times
JULY 7, 2023
Second dose of treatment generates response among young people during Oxford University trial - News - PharmaTimes

Pharmaceutical Technology
JULY 7, 2023
The US FDA has awarded priority review for Astellas Pharma’s biologics licence application for zolbetuximab to treat gastric cancer.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

XTalks
JULY 7, 2023
Milestone Pharmaceuticals Inc., a Canadian biopharmaceutical firm specializing in cardiovascular therapies, recently reported positive results from a Phase III clinical trial of its leading candidate, etripamil nasal spray, for the treatment of paroxysmal supraventricular tachycardia (PSVT). The trial results, published in The Lancet , demonstrated significant improvement in various PSVT symptoms among patients who received the nasal spray compared to those who received a placebo.

Pharmaceutical Technology
JULY 7, 2023
ABBV-101 is under clinical development by AbbVie and currently in Phase I for Relapsed Chronic Lymphocytic Leukemia (CLL).

Outsourcing Pharma
JULY 7, 2023
Anorexia nervosa is a notoriously difficult illness to treat. It has the highest mortality rate of any psychiatric disorder, yet there is currently no FDA approved therapy for the condition.

Pharmaceutical Technology
JULY 7, 2023
ABBV-101 is under clinical development by AbbVie and currently in Phase I for Relapsed Chronic Lymphocytic Leukemia (CLL).

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

XTalks
JULY 7, 2023
Earlier this week, the World Health Organization (WHO) released new guidelines that emphasized the critical role that marketers must play in safeguarding children’s health. The WHO underscored the need for tighter regulations to protect children from the adverse effects of food marketing. This directive of marketing food to children carries profound implications, demanding a significant shift in child-focused marketing strategies.

Pharmaceutical Technology
JULY 7, 2023
The US FDA has granted traditional approval for Eisai and Biogen’s Leqembi to treat Alzheimer’s disease (AD) in adults.
Fierce Pharma
JULY 7, 2023
AstraZeneca and Daiichi Sankyo's TROP2 antibody-drug conjugate returned a concerning safety message. Moderna reportedly plans to invest up to $1 billion in China. | AstraZeneca and Daiichi Sankyo delivered a concerning safety message for their TROP2 antibody-drug conjugate. Moderna reportedly plans to invest up to $1 billion in China. Takeda expanded its antibody partnership with F-Star Therapeutics with a new deal potentially worth $1 billion.
Pharmaceutical Technology
JULY 7, 2023
Shorla Oncology has signed a licensing agreement with an undisclosed drug UK firm for a chemotherapy drug, PIP-101.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharma Times
JULY 7, 2023
Therapy involves the treatment of non-segmental vitiligo among adults and adolescents - News - PharmaTimes
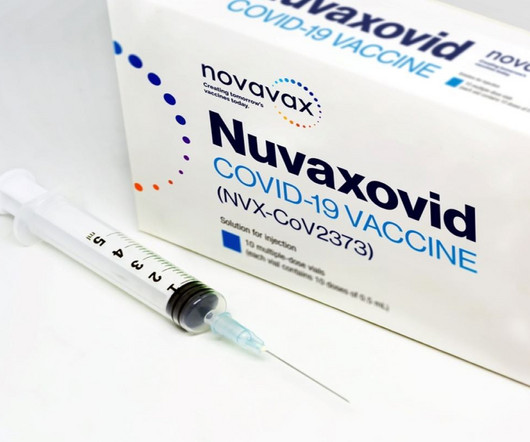
Pharmaceutical Technology
JULY 7, 2023
The EMA has granted Novavax’s Covid-19 vaccine a full marketing authorisation, based on Phase III trial data.

Drug Channels
JULY 7, 2023
Today’s guest post comes from Sahil Naik, Chief of Staff to the CEO at Phil, Inc. Sahil discusses how manufacturers can balance patients’ access to therapy with gross-to-net (GTN) goals. Visit [link] to learn more about how Phil helps improve the patient experience while managing GTN. Read on for Sahil’s insights. Read more » Copyright © 2006-2023 Pembroke Consulting, Inc. d/b/a Drug Channels Institute.
Pharmaceutical Technology
JULY 7, 2023
Pfizer has invested $25m in clinical-stage CRISPR genome-editing biopharmaceutical firm Caribou Biosciences.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Fierce Pharma
JULY 7, 2023
After a pandemic-fueled lull the past few years, FDA inspections are back in full force. This week, a pair of Indian drugmakers have found themselves in the agency’s crosshairs. | The FDA has issued a trio of Form 483s after inspecting three Indian drug plants. One write-up went to Intas Pharmaceuticals while the other two were handed down to Ipca Laboratories.

Pharmaceutical Technology
JULY 7, 2023
Oncolytic Virus for Human Papillomavirus Associated Cancer, Human Papillomavirus Infections and Coronavirus Disease 2019 (COVID-19) is under clinical development by Blue Sky Immunotherapies and currently in Phase I for Cervical Intraepithelial Neoplasia (CIN).

pharmaphorum
JULY 7, 2023
RxPreferred to offer Humira biosimilar through Mark Cuban's online pharmacy Eloise.

Pharmaceutical Technology
JULY 7, 2023
Oncolytic Virus for Human Papillomavirus Associated Cancer, Human Papillomavirus Infections and Coronavirus Disease 2019 (COVID-19) is under clinical development by Blue Sky Immunotherapies and currently in Phase I for Cervical Intraepithelial Neoplasia (CIN).

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
JULY 7, 2023
Changing Faces: Pharma and biotech hires from May & June 2023 Mike.

Pharmaceutical Technology
JULY 7, 2023
ORIN-1001 is under clinical development by Shanghai Fosun Pharmaceutical (Group) and currently in Phase II for Kidney Cancer (Renal Cell Cancer).

pharmaphorum
JULY 7, 2023
America’s mental health crisis: Why we need a Moonshot Mike.

Pharmaceutical Technology
JULY 7, 2023
ORIN-1001 is under clinical development by Shanghai Fosun Pharmaceutical (Group) and currently in Phase II for Kidney Cancer (Renal Cell Cancer).

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content