Annovis looks to advance pivotal Alzheimer’s and Parkinson’s drug studies
Pharmaceutical Technology
OCTOBER 25, 2023
The AD and PD drug aims to reduce the level of neurotoxic proteins, thereby improving information flow across the axonal transport
Pharmaceutical Technology
OCTOBER 25, 2023
The AD and PD drug aims to reduce the level of neurotoxic proteins, thereby improving information flow across the axonal transport
Bio Pharma Dive
OCTOBER 25, 2023
An under-the-skin injection of Leqembi performed about the same as the already marketed intravenous form, according to trial results presented Wednesday. A new approval application is expected by the end of March.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
OCTOBER 25, 2023
iCARE4CVD is coordinated by Novo Nordisk and aims to collect and analyse data from 1 million people to validate personalized treatments.

Bio Pharma Dive
OCTOBER 25, 2023
“They have the money” and they’re using it to influence drug development, according to the director of the IQVIA Institute for Human Data Science.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 25, 2023
The Division of Non Communicable Diseases (NCD) of the Indian Council of Medical Research (ICMR) has come out with a Consensus Document for Management of Prostate Cancer to equip clinicians and students on latest therapies.
Bio Pharma Dive
OCTOBER 25, 2023
The CDC is asking doctors to ration supply of Sanofi and AstraZeneca’s new RSV drug Beyfortus, as demand has outstripped supply.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Bio Pharma Dive
OCTOBER 25, 2023
The study results, while only from three participants, offer a glimpse at how one of the few in vivo CRISPR therapies in U.S. human testing is working.

Pharmaceutical Technology
OCTOBER 25, 2023
AxoSim’s microBrain drug discovery platform acquisition includes a US manufacturing facility, and associated patents and IP.

Bio Pharma Dive
OCTOBER 25, 2023
The latest round of cuts brings the company’s total announced workforce reductions this year to more than 1,000.

Pharmaceutical Technology
OCTOBER 25, 2023
Experts discuss the key trends in quality improvements and API reshoring for the generic drugs market at CpHI Europe.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
OCTOBER 25, 2023
This podcast series explores how life sciences companies can bolster their cybersecurity while growing their business.
Pharmaceutical Technology
OCTOBER 25, 2023
The US FDA has granted approval for Servier’s Tibsovo to treat IDH1-mutated relapsed or refractory (R/R) myelodysplastic syndromes (MDS).

Pharma Times
OCTOBER 25, 2023
The APPG has called for urgent improvements in rural and ethnically diverse-areas - News - PharmaTimes

Pharmaceutical Technology
OCTOBER 25, 2023
Novartis has posted net income of $1.51bn in the third quarter (Q3) of 2023, a 14% rise compared to $1.33bn in the same quarter of 2022.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

BioSpace
OCTOBER 25, 2023
Attributing losses to exchange rate and pipeline impairment losses, the Japanese biopharma dropped its reported net profit forecast of 142 billion yen ($945.1 million) to 93 billion yen ($618.8 million).

Pharmaceutical Technology
OCTOBER 25, 2023
Insmed and Google Cloud have partnered to leverage generative AI for transforming the discovery, development and marketing of drugs.

Drug Patent Watch
OCTOBER 25, 2023
DrugPatentWatch has published a list of research papers citing DrugPatentWatch at [link] DrugPatentWatch.com is your key to unlocking the world of pharmaceutical research. These primary literature citations can benefit researchers,… The post List of research papers citing DrugPatentWatch appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
OCTOBER 25, 2023
Sitravatinib malate is under clinical development by Mirati Therapeutics and currently in Phase II for Metastatic Melanoma.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

BioSpace
OCTOBER 25, 2023
Traditional ideas of what attracts talent have, in many ways been upended, with some locations faring better than others. And what employees look for in a job is still changing.

Pharmaceutical Technology
OCTOBER 25, 2023
Sitravatinib malate is under clinical development by Mirati Therapeutics and currently in Phase II for Metastatic Melanoma.

Pharma Times
OCTOBER 25, 2023
The company will launch a clinical investigation of EA-2351 for geographic atrophy - News - PharmaTimes
Pharmaceutical Technology
OCTOBER 25, 2023
BMS has received breakthrough therapy designation from the US FDA for its investigational therapy BMS-986278 to treat PPF.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Fierce Pharma
OCTOBER 25, 2023
After misconduct at a key trial site raised questions of | The FDA previously uncovered evidence of forged emails and trial misconduct in the company's phase 3 Alzheimer's disease agitation study. Now, with a third-party audit in hand, BioXcel looks to push forward with the drug.

Pharmaceutical Technology
OCTOBER 25, 2023
Aiolos will lead the anti-TSLP antibody into Phase II clinical trials after demonstrating high potency in Phase I study.
BioSpace
OCTOBER 25, 2023
A third-party audit found no integrity and reliability problems with data from BioXcel Therapeutics’ Phase III trial. The company intends to file a supplemental New Drug Application for its candidate BXCL501.
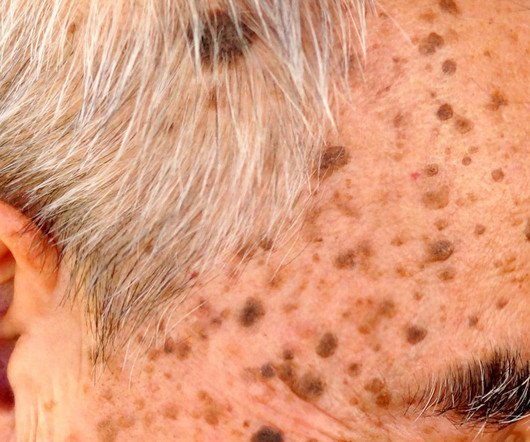
Pharmaceutical Technology
OCTOBER 25, 2023
The funds will go towards Phase II trials for the company’s two candidates treating seborrheic keratoses and melasma.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
OCTOBER 25, 2023
Following the regulator’s denial of patisiran’s label expansion, Alnylam has published late-stage data for the RNAi therapeutic in The New England Journal of Medicine demonstrating its efficacy in ATTR-cardiomyopathy.

Pharmaceutical Technology
OCTOBER 25, 2023
MEN-1703 is under clinical development by Stemline Therapeutics and currently in Phase II for Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia).

Outsourcing Pharma
OCTOBER 25, 2023
Advanced tech company, PhaseV, has raised $15 million in funding that it says it will use to âpush the boundaries of machine learning for clinical trial optimizationâ.

Pharmaceutical Technology
OCTOBER 25, 2023
MEN-1703 is under clinical development by Stemline Therapeutics and currently in Phase II for Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia).

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content