PepGen cleared by FDA to begin study of muscular dystrophy drug
Bio Pharma Dive
OCTOBER 12, 2023
The company expects to deliver first results next year from the early-stage study of people with myotonic dystrophy type 1.

Bio Pharma Dive
OCTOBER 12, 2023
The company expects to deliver first results next year from the early-stage study of people with myotonic dystrophy type 1.
AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 12, 2023
An elderly woman in Russia’s Far East was revealed on Wednesday to have lived for 80 years with an inch-long needle in her brain, after doctors made the unexpected discovery during a CT scan.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 12, 2023
IMIDomics has entered into a licence and partnership agreement with the University of Barcelona (UB) to advance treatments for IMIDs.

Rethinking Clinical Trials
OCTOBER 12, 2023
In a new article published this week in Contemporary Clinical Trials Communications, the GRACE Demonstration Project team recommends that suicidality should be monitored in pragmatic clinical trials that measure depression as an outcome. The work builds on their experience conducting research involving patients with sickle cell disease and on previous work from the NIH Pragmatic Trials Collaboratory’s Ethics and Regulatory Core.

Bio Pharma Dive
OCTOBER 12, 2023
Earlier this year Pfizer removed thousands of participants from a study of its Lyme disease shot over concerns the group, Care Access, wasn't meeting clinical practice standards.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 12, 2023
A Parliamentary Panel that looked into the implementation of Pradhan Mantri TB Mukt Bharat Abhiyan (PMTBMBA) has advised the Centre to use latest technologies such as drones, data integration systems and mobile apps to strengthen surveillance in order to test, control and eradicate tuberculosis from the country.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
OCTOBER 12, 2023
Leading parenteral packaging company Datwyler has extended its range of coating options with UltraShield™, a high-performance film-coated solution for safely storing innovative drugs.

Pharma Times
OCTOBER 12, 2023
The financing will support the biotech’s clinical study of people with AD as a lead programme - News - PharmaTimes

Pharmaceutical Technology
OCTOBER 12, 2023
Cellares entered an expanded agreement with Bristol Myers Squibb to provide proof-of-concept manufacturing for a second CAR-T cell therapy.

BioSpace
OCTOBER 12, 2023
The biotech will pause its lirafugratinib program for a rare bile duct cancer to target a larger FGFR2-altered solid tumors population, citing the Inflation Reduction Act as a driving decision factor.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
OCTOBER 12, 2023
The Phase III trial of ALK’s allergy immunotherapy tablet met its primary endpoint for the treatment of tree-pollen-induced allergics.
Fierce Pharma
OCTOBER 12, 2023
An apparent win for Novo Nordisk’s GLP-1 blockbuster Ozempic in chronic kidney disease (CKD) could herald a shift in how the condition has been treated for decades. | Based on the inclusion criteria for Novo Nordisk’s chronic kidney disease trial, dialysis bigwig DaVita believes there may only be “limited application” of the study’s findings to the overall CKD population.
Pharmaceutical Technology
OCTOBER 12, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has published updated guidance on valproate-containing medicines.

Intouch Solutions
OCTOBER 12, 2023
To wrap up Q3, our U.S.-based offices celebrated in person with an End of Summer Fair complete with nostalgic carnival treats and games, caricatures, a photo booth and more! Most importantly, these gatherings gave us time away from our desks to spend time with one another. At EVERSANA INTOUCH we value building relationships with one another and keeping our one-of-a-kind culture alive.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
OCTOBER 12, 2023
Researchers at the University of Birmingham have developed antibody fragments, called nanobodies, in a bid to understand platelet disorders such as thrombosis.

Fierce Pharma
OCTOBER 12, 2023
Even as Teva Pharmaceuticals’ Icelandic counterpart Alvotech works to get its Reykjavik manufacturing facility back up to snuff, lingering problems at the plant—which derailed approval of the partn | The FDA shot down Alvotech and Teva's biosimilar candidate to Johnson & Johnson's Stelara over “certain deficiencies” uncovered by the FDA during a reinspection of the company's factory in Iceland earlier this year.

Pharmaceutical Technology
OCTOBER 12, 2023
Almirall will license EpimAb’s Fabs-In-Tandem Immunoglobulin (FIT-Ig) platform to develop up to three bispecific antibody targets.

BioSpace
OCTOBER 12, 2023
The layoffs, set to take effect in late November, will impact about a third of Reata’s headcount. The workforce reduction comes just weeks after Biogen completed its $7.3 billion Reata buy.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
OCTOBER 12, 2023
Care Access has been exonerated of wrongdoing at its clinical sites, but Pfizer and Valneva’s Phase III trial is now one year behind schedule.

BioSpace
OCTOBER 12, 2023
Dialysis giant DaVita on Thursday raised concerns over Novo Nordisk’s interim analysis of a kidney outcomes study of semaglutide, pointing to the potentially limited applicability of the findings.
Pharmaceutical Technology
OCTOBER 12, 2023
Astria Therapeutics has signed a global exclusive agreement to license Ichnos Sciences’ OX40 portfolio for treating atopic dermatitis (AD).

Pharma Times
OCTOBER 12, 2023
The service will benefit major research and industry projects across the UK - News - PharmaTimes

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Pharmaceutical Technology
OCTOBER 12, 2023
A large proportion of chronic HF patients are taking suboptimal doses of relevant medications, leading to a worsening of symptoms.
BioPharma Reporter
OCTOBER 12, 2023
Terumo Blood and Cell Technologies, a medical tech company, and BioCentriq, a cell therapy contract development and manufacturing organization (CDMO), are working together to demonstrate the capabilities of Terumoâs automated cell and gene therapy platforms to accelerate CAR-T cell development.
Pharmaceutical Technology
OCTOBER 12, 2023
The institute aims to use these nanobodies to develop validated clinical assays for testing patients with platelet disorders.

BioSpace
OCTOBER 12, 2023
More than 54% of patients treated with mirikizumab achieved clinical remission at 52 weeks versus 19.6% of those on placebo. Eli Lilly will submit a marketing application in Crohn’s disease to the FDA in 2024.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
OCTOBER 12, 2023
Immetas Therapeutics and GC Biopharma announced a collaboration for mRNA therapeutic development.
BioSpace
OCTOBER 12, 2023
In a Phase Ib/II study, Tempest Therapeutics’ investigational PPAR⍺ antagonist—combined with Roche’s Avastin and Tecentriq—showed strong signs of survival benefits in liver cancer patients.
Pharmaceutical Technology
OCTOBER 12, 2023
The investment will ensure that 370 million children in low-income countries will be vaccinated against polio each year.
BioSpace
OCTOBER 12, 2023
The biopharma’s data manipulation controversy continues with a recently leaked City University of New York report, which found signs of “egregious” and “deliberate” misconduct by a company advisor.
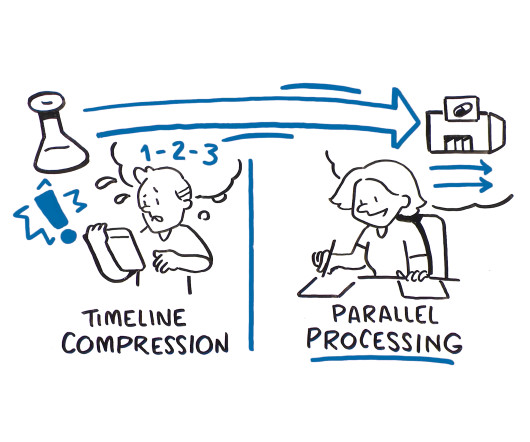
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content