FDA approves Takeda’s Adzynma for rare blood clotting disorder
Pharmaceutical Technology
NOVEMBER 10, 2023
The US FDA has granted approval for Takeda’s Adzynma for congenital thrombotic thrombocytopenic purpura, a rare blood clotting disorder.

Pharmaceutical Technology
NOVEMBER 10, 2023
The US FDA has granted approval for Takeda’s Adzynma for congenital thrombotic thrombocytopenic purpura, a rare blood clotting disorder.

Bio Pharma Dive
NOVEMBER 10, 2023
Cargo Therapeutics is the seventh oncology biotech to successfully price an IPO this year.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
NOVEMBER 10, 2023
AstraZeneca has posted a profit after tax of $1.37bn for Q3 2023 versus $1.64bn in the same quarter of the previous year, a decline of 16%.

Worldwide Clinical Trials
NOVEMBER 10, 2023
Pain for chronic patients is not merely a condition they must manage but a social dynamic with various feelings and opinions. Its psychological impact is often understated, though it carries the same weight as pain’s physical effects. At this year’s annual Pain Therapeutics Summit , which took place in San Diego, CA, October 19 to 20th, I heard brilliant presentations on novel pain medication development, targeted discoveries, pain classifications, the NIH HEAL Initiative , trial designs, biomar

Bio Pharma Dive
NOVEMBER 10, 2023
The maker of Wegovy and Ozempic plans to expand production in its home country of Denmark to help meet surging demand for the GLP-1 drugs.

Worldwide Clinical Trials
NOVEMBER 10, 2023
As someone who joined the military right after high school, I can say that it was one of the best decisions I ever made. My nearly perfect score on the Armed Services Vocational Aptitude Battery Test (ASVAB) qualified me for three positions, and I ended up taking the one that required a top-secret/SCI security clearance – as a Record Telecommunications Center Operator.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
NOVEMBER 10, 2023
Following the three-month FDA review delay, Valneva’s live-attenuated chikungunya vaccine, Ixchiq received accelerated approval.
Pharma Times
NOVEMBER 10, 2023
The trial is testing whether Mavenclad could benefit upper limb movement in MS patients - News - PharmaTimes

Pharmaceutical Technology
NOVEMBER 10, 2023
Novartis expects final Phase III REMIX trial readouts and regulatory submissions for remibrutinib in 2024.

Outsourcing Pharma
NOVEMBER 10, 2023
A partnership between clinical research technology company uMotif and cognitive science company, Cogstate Limited, will see the companies continue existing work that includes a major phase 2 clinical trial of a psychedelic therapeutic.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
NOVEMBER 10, 2023
The EC has granted approval for Daiichi Sankyo’s Vanflyta to treat FLT3-ITD positive acute myeloid leukaemia (AML) in adults.

Pharma Times
NOVEMBER 10, 2023
A rare disease is defined as a condition that affects fewer than one in 2,000 people - News - PharmaTimes

Pharmaceutical Technology
NOVEMBER 10, 2023
Bayer has expanded a drug discovery research partnership with Recursion Pharmaceuticals in the precision oncology sector.
Outsourcing Pharma
NOVEMBER 10, 2023
Excessive costs cost and time to market in clinical trials could soon be eliminated thanks to the addition of a patient access store (PAS) to Phesi's trial accelerator platform.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
NOVEMBER 10, 2023
If approved, Breyanzi would become the first and only CAR T cell therapy available for this specific patient population.

Outsourcing Pharma
NOVEMBER 10, 2023
The first scientific milestone stemming from a collaboration between Sosei Heptares and Kallyope that was announced last year (2022) has been reached.

Pharmaceutical Technology
NOVEMBER 10, 2023
At the BIO-Europe conference, experts discussed remaining challenges in AI healthcare interventions.

Fierce Pharma
NOVEMBER 10, 2023
Valneva has won the race in the U.S. | The FDA has approved the world’s first chikungunya vaccine, giving a thumbs-up to Valneva’s Ixchiq. The French company receives a priority review voucher from the FDA, which it said it will sell. It is an accelerated approval and subject to a confirmatory, real-world study.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Pharmaceutical Technology
NOVEMBER 10, 2023
Amidst POINT’s shareholder turbulence, Lilly is keen to move forward with the $1.4bn acquisition.
Drug Patent Watch
NOVEMBER 10, 2023
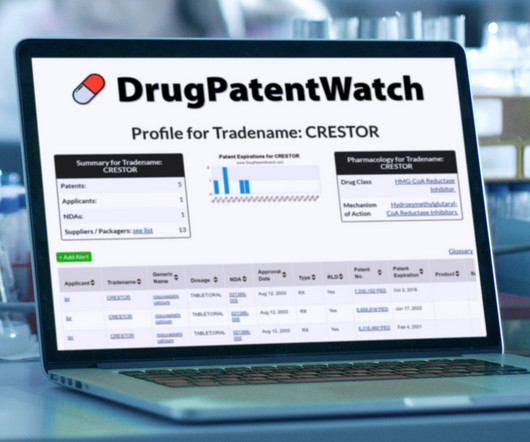
Acetylcysteine is the generic ingredient in seven branded drugs marketed by Cumberland Pharms, Aspen, Eugia Pharma, Exela Pharma, Fresenius Kabi Usa, Indoco, Rising, Sagent Pharms Inc, Steriscience, Zydus Pharms, Alvogen,… The post New tentative approval for Rising drug acetylcysteine appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
NOVEMBER 10, 2023
The advent of messenger RNA (mRNA) therapeutics has revolutionized modern medicines.

Drug Channels
NOVEMBER 10, 2023
Today’s guest post comes from James Pisano, Partner, Market Access at The Dedham Group and Dinesh Kabaleeswaran, SVP of Insights & Advisory Teams at MMIT. James and Dinesh offer four trends that they predict will shape medication access in the coming years. Click here to learn about consulting services from MMIT and its sister company The Dedham Group.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

pharmaphorum
NOVEMBER 10, 2023
Bayer pledges up to $1.5bn for Recursion oncology alliance Phil.

XTalks
NOVEMBER 10, 2023
The US Food and Drug Administration (FDA) has approved Takeda Pharmaceuticals’ Adzynma, the first recombinant protein product for prophylactic (preventive) or on‑demand enzyme replacement therapy (ERT) in adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP), an ultra-rare blood clotting disorder. Adzynma is administered intravenously once every other week for prophylactic ERT and once daily for on-demand ERT. cTTP is a very rare, inherited and life-threatening
pharmaphorum
NOVEMBER 10, 2023
Novo Nordisk sets aside $6bn to boost production capacity Phil.

Fierce Pharma
NOVEMBER 10, 2023
Mirati Therapeutics’ KRAS inhibitor Krazati’s EU prospects weren’t looking good after an initial rejection from Europe's drugs regulator. | European Medicines Agency’s (EMA's) Committee for Medicinal Products for Human Use (CHMP) snubbed the KRAS inhibitor in July, but changed its tune after a formal re-examination initiated by Mirati.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
NOVEMBER 10, 2023
Takeda gets okay in US for rare blood disorder drug Phil.
Pharmaceutical Commerce
NOVEMBER 10, 2023
New E2E workflow orchestration service is expected to simplify the processes of health systems and retail pharmacies while also reducing the risk of recalled items reaching patients.

Drug Discovery World
NOVEMBER 10, 2023
Topline results from a Phase I/II clinical trial have shown that Kiora’s light-restoring small molecule KIO-301 has the potential to meaningfully improve vision in patients with retinitis pigmentosa (RP). The results of the ABACUS study for Kiora’s intravitreal (IVT) molecular photoswitch were presented at the American Academy of Ophthalmology annual conference by Russell Van Gelder, Professor and Chair, Department of Ophthalmology, University of Washington, School of Medicine.

Antidote
NOVEMBER 10, 2023
In 2018, we partnered with SCORR Marketing to conduct a survey aimed at gaining knowledge about the patient perception of clinical trial participation. We collected nearly 4,000 responses from individuals regarding what matters most to when considering a clinical trial.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content