Hyloris wins painkiller approval amidst amplified anti-opioid efforts
Pharmaceutical Technology
OCTOBER 18, 2023
The FDA approved Hyloris’s non-opioid painkiller as the agency increases efforts to mitigate an opioid crisis.
Pharmaceutical Technology
OCTOBER 18, 2023
The FDA approved Hyloris’s non-opioid painkiller as the agency increases efforts to mitigate an opioid crisis.

Bio Pharma Dive
OCTOBER 18, 2023
A panel of FDA advisers last month found BrainStorm’s data unconvincing. The company now says a Phase 3b study will be needed for its NurOwn cell therapy to have a shot at an approval.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
OCTOBER 18, 2023
Lisata has received orphan drug designation from the EMA's Committee for Orphan Medicinal Products for LSTA1 to treat pancreatic cancer.
Bio Pharma Dive
OCTOBER 18, 2023
CEO Emil Kakkis says the company's findings are too exciting to ignore, but the “high-risk, high-return” venture needs to be pursued outside the organization.

Pharmaceutical Technology
OCTOBER 18, 2023
The company also secured $50m in debt financing to support the commercial launch of Xphozah in November 2023.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 18, 2023
The most widely prescribed class of antidepressant has – at long last – been shown to increase connections in the human brain. The discovery offers a plausible biological explanation for the medication’s delayed treatment response and could help develop new targeted treatments.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Rethinking Clinical Trials
OCTOBER 18, 2023
In this Friday’s PCT Grand Rounds, Rachael Fleurence of the NIH and Joshua Sharfstein of Johns Hopkins University will present “A National Initiative to Eliminate Hepatitis C in the United States – Why This Matters to Clinical Trialists.” The Grand Rounds session will be held on Friday, October 20, 2023, at 1:00 pm eastern. Fleurence is a senior advisor at the NIH.

Bio Pharma Dive
OCTOBER 18, 2023
Developed with Regeneron, Intellia’s treatment is designed to inactivate a gene to treat an inherited disease called transthyretin amyloidosis.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 18, 2023
The National Institute of Pharmaceutical Education and Research (NIPER) has underlined indispensability of artificial intelligence in pharmaceutical research. The applications of AI have enabled faster, more cost-effective, and data-driven approaches across the drug discovery and development lifecycle.

Pharmaceutical Technology
OCTOBER 18, 2023
Concerns have arisen that innovative technologies such as artificial intelligence (AI) could have a damaging effect on HCP relationships

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
OCTOBER 18, 2023
The biotech plans to hire additional sales staff to support the U.S. launch of Xphozah, which had been rejected by the FDA two years ago.
Pharmaceutical Technology
OCTOBER 18, 2023
Sage Therapeutics’ SAGE-718 has been awarded the orphan drug designation from the US Food and Drug Administration (FDA) for the treatment of Huntington’s Disease.

Rethinking Clinical Trials
OCTOBER 18, 2023
Speaker Keith Marsolo, PhD Associate Professor Department of Population Health Sciences Duke University School of Medicine Slides Keywords PCORnet, Common Data Model, EHR, Social Determinants of Health Key Points There are many different definitions of social determinants of health. The World Health Organization defines social determinants of health as non-medical factors that influence health outcomes and conditions in which people are born, grow, work, live, and age, and the wider set of force
Pharmaceutical Technology
OCTOBER 18, 2023
The positive opinion expands the antiviral drug’s use to CMV-positive donors and CMV-negative kidney transplant patients at high risk of CMV infection

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
OCTOBER 18, 2023
Editor's Note: Please check back on Oct. 22 when the full data of EV-302 are presented at ESMO 2023. | The results are in for a phase 3 bladder cancer trial assessing the combination of Seagen and Astellas' Padcev, plus Merck's Keytruda, versus standard of care chemotherapy. The figures indicate that the combo can be transformative in the indication.
Pharmaceutical Technology
OCTOBER 18, 2023
The data from the Phase II trial showed the efficacy of tipifarnib in patients with head and neck squamous cell carcinoma.

BioSpace
OCTOBER 18, 2023
The contracting COVID-19 market has led to lower third-quarter sales for the Swiss drugmaker, which made the decision to eliminate four clinical programs following underwhelming readouts.

Pharmaceutical Technology
OCTOBER 18, 2023
Gilead Sciences has entered a partnership with Assembly Biosciences to advance the research and development of new antiviral therapies.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

BioSpace
OCTOBER 18, 2023
Early stage ALK-positive non-small cell lung cancer patients treated with Roche’s Alecensa saw an “unprecedented” 76% drop in the risk of recurrence or death, the company announced Wednesday.

Pharmaceutical Technology
OCTOBER 18, 2023
Monte Rosa Therapeutics and Roche have signed an agreement for the development of MGDs to target cancer and neurological diseases.

Fierce Pharma
OCTOBER 18, 2023
Pfizer will cough up $2 million to settle allegations from the U.S. Department of Labor that it underpaid certain women who were employed at the company’s New York City headquarters. | The accusations centered on the company's compensation of 86 female employees in 2015 and 2016. Under the settlement with the Department of Labor, the company must also set aside $500,000 for potential future salary adjustments.

Pharmaceutical Technology
OCTOBER 18, 2023
The FDA has pushed Livmarli’s PDUFA date to 13 March 2024, citing an information request deemed a major amendment.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharma Times
OCTOBER 18, 2023
UK researchers can apply to receive a share of £3m to set up transdisciplinary networks - News - PharmaTimes

BioSpace
OCTOBER 18, 2023
The trial, which is the first late-stage study of an in vivo CRISPR treatment in the U.S., will start by the end of 2023. Intellia's NTLA-2001 is a treatment candidate for transthyretin amyloidosis cardiomyopathy.

Pharmaceutical Commerce
OCTOBER 18, 2023
These components take the burden off drug manufacturers to prep packaging components for sterilization, while helping to enhance the drug filling and packaging process.
BioSpace
OCTOBER 18, 2023
Following a delay and an initial rejection, UCB’s IL-17A/IL-17F blocker bimekizumab was approved for moderate-to-severe plaque psoriasis, which will now be marketed with the brand name Bimzelx.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioPharma Reporter
OCTOBER 18, 2023
Bioiberica says the excitement for this yearâs CPHI has been âtaken up a notchâ as the company looks forward to welcoming both old and new customers to its hometown, Barcelona.

ProRelix Research
OCTOBER 18, 2023
The increasing focus and acceptance of real-world data (RWD) in clinical decision making has led regulatory authorities and sponsors to understand its influence on data management that is an integral […] The post The Impact of Real-World Evidence on Clinical Data Management appeared first on ProRelix Research.

BioPharma Reporter
OCTOBER 18, 2023
Emma Harvey is global head of medical affairs at F2G Ltd, a UK and Austria based biotech where she is responsible for the global medical and commercial strategies for a novel antifungal drug for serious systemic infections, in clinical development.

BioSpace
OCTOBER 18, 2023
The deal with PTC Therapeutics increases Royalty Pharma’s stake to 13% of total royalties garnered by Evrysdi, the blockbuster drug for spinal muscular atrophy licensed and marketed by Roche.
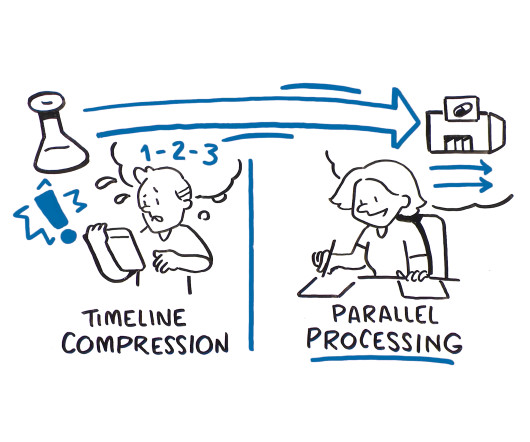
Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content