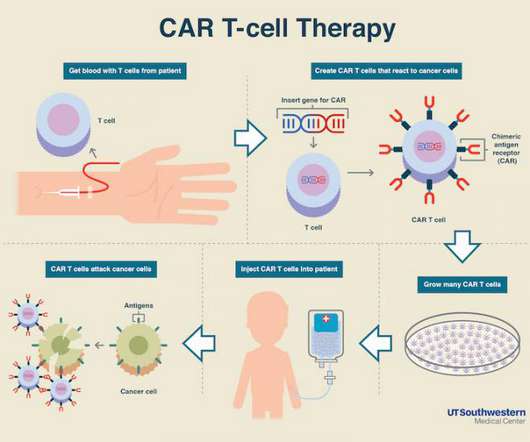
America’s healthcare costs are in deep crisis
World of DTC Marketing
MARCH 8, 2021
SUMMARY: The media loves to blame pharma companies for high healthcare costs, but unless we start to invest in healthy lifestyles, we’re headed for a healthcare crisis the likes of which we have never experienced. The media have targeted pharmaceutical companies for a long time because sensational headlines lead to clicks. Forget the fact that prescription drugs only account for $.10 of every healthcare dollar spent and that hospital chains are raking in cash with PBM’s and insurers



































Let's personalize your content