Moderna claims positive results in early study for combo COVID, flu shot
Bio Pharma Dive
OCTOBER 4, 2023
The company is planning to start a Phase 3 trial of the vaccine this year, and is targeting a regulatory approval in 2025.

Bio Pharma Dive
OCTOBER 4, 2023
The company is planning to start a Phase 3 trial of the vaccine this year, and is targeting a regulatory approval in 2025.
AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 4, 2023
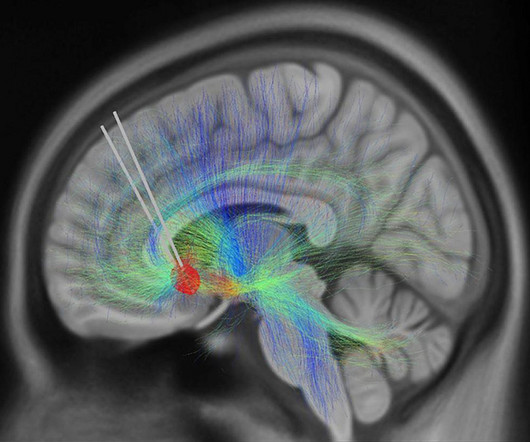
It could soon be possible to measure changes in depression levels like we can measure blood pressure or heart rate. In a new study, 10 patients with depression that had resisted treatment were enrolled in a six-month course of deep brain stimulation (DBS) therapy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 4, 2023
Strides earned market approval in the US as part of a larger government endeavor to address HIV worldwide.

Rethinking Clinical Trials
OCTOBER 4, 2023
In this Friday’s PCT Grand Rounds, David Murray of the NIH Office of Disease Prevention will kick off our special series, Advances in the Design and Analysis of Pragmatic Clinical Trials, with his presentation, “Hybrid Studies Should Not Sacrifice Rigorous Methods.” The session will be held on Friday, October 6, at 1:00 pm eastern and will be moderated by Jonathan Moyer.

Bio Pharma Dive
OCTOBER 4, 2023
The spinoff, now complete, is a major step in CEO Vas Narasimhan’s plan to refocus Novartis more tightly around novel prescription drugs and new technologies.
Pharmaceutical Technology
OCTOBER 4, 2023
The US FDA has amended the EUA of Novavax’s adjuvanted Covid-19 vaccine (NVX-CoV2601) to incorporate the 2023-2024 formula.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
OCTOBER 4, 2023
The pharma will pay $500 million to collaborate with Teva on the anti-TL1A therapy, which is seen as a competitor to similar drugs from Merck and Roivant.

Pharmaceutical Technology
OCTOBER 4, 2023
The voluntary recall issued by Hospira will affect three different injectables following the potential presence of glass particles.

Bio Pharma Dive
OCTOBER 4, 2023
Company veteran Mike Mason, who took leadership of the division four years ago, is retiring and will be replaced by immunology head Patrik Jonsson.

Pharmaceutical Technology
OCTOBER 4, 2023
Ernst and Young’s latest report finds supply chain visibility to be a viable route for pharmaceutical resilience.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharma Times
OCTOBER 4, 2023
The companies aim to bring gene therapies to patients sooner than traditional approaches - News - PharmaTimes

Pharmaceutical Technology
OCTOBER 4, 2023
Novartis will now focus on innovative drugs, whilst Sandoz strengthens its biosimilar business.

Fierce Pharma
OCTOBER 4, 2023
After more than a year of preparation, Sandoz has officially parted ways with Swiss drug giant Novartis. But the company's day-one valuation lagged what some analysts had projected. | The generics and biosimilar maker debuted on the SIX Swiss Exchange at 24 Swiss francs ($26.16) for a total valuation of about $11.2 billion. Analysts had previously published ranges of $11 billion to $26 billion for the company, according to Reuters.

Pharmaceutical Technology
OCTOBER 4, 2023
Regeneron has entered into an expanded research collaboration with Intellia Therapeutics for the development of CRISPR based therapies.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Fierce Pharma
OCTOBER 4, 2023
As long expected, the U.S. government is winding down its distribution of COVID-19 countermeasures. | The government will wind down its Lagevrio distribution ahead of the transition, the Administration for Strategic Preparedness and Response said.

Pharmaceutical Technology
OCTOBER 4, 2023
Eli Lilly and Company has signed a definitive agreement to acquire POINT Biopharma for an all-in cash deal value of nearly $1.4bn.

XTalks
OCTOBER 4, 2023
Health economics and outcomes research (HEOR) and market access strategies play a critical role in ensuring that newly approved therapies are made available to patients. Professionals in these roles are facing new challenges when it comes to negotiating coverage for many of the new, innovative treatments that are coming to market. HEOR involves the assessment of the economic implications and real-world outcomes of cutting-edge therapies, providing essential insights into their cost-effectiveness

Pharmaceutical Technology
OCTOBER 4, 2023
The WHO SAGE has recommended Takeda’s QDENGA (dengue tetravalent vaccine) for use in high dengue burden and transmission areas.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
OCTOBER 4, 2023
Boehringer Ingelheim, which recently launched the first interchangeable biosimilar to AbbVie’s megablockbuster Humira, is getting in on the industry’s double-pricing trick. | After debuting its branded Humira biosim Cyltezo in July, Boehringer has launched an unbranded copycat of the inflammatory disease drug, simply called adalimumab-adbm injection, which will be sold at an 81% discount to Humira.

Pharmaceutical Technology
OCTOBER 4, 2023
The optical imaging agent, FG001, is being evaluated for visualising the malignant tissue during high-grade glioma surgery.
BioSpace
OCTOBER 4, 2023
Data from the Mayo Clinic shows limited eligibility for the anti-amyloid treatment. However, Michael Irizarry, Eisai’s deputy chief clinical officer, says some patients could still be eligible.

Pharmaceutical Technology
OCTOBER 4, 2023
The agreement provides Teva with a $500m upfront payment along with potential milestone-based payments of up to $1bn.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

BioSpace
OCTOBER 4, 2023
The Japan-based pharma is acquiring Orchard Therapeutics for approximately $477.6 million, if all conditions are met. Orchard’s pediatric gene therapy has a PDUFA date set for March 2024.

Fierce Pharma
OCTOBER 4, 2023
Lilly announced several C-Suite moves, including the retirement of Diabetes and Obesity chief Mike Mason, with his duties assumed by Patrick Jonsson.

BioSpace
OCTOBER 4, 2023
Despite the lack of a randomized controlled trial for the US WorldMeds’ investigational drug, an FDA advisory committee found that the company provided adequate data to support its benefit in high-risk neuroblastoma.

Pharma Times
OCTOBER 4, 2023
The approach reduces recovery times and lowers rates of complications - News - PharmaTimes

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
pharmaphorum
OCTOBER 4, 2023
Novavax has been given the go-ahead by regulators in the US for an updated version of its COVID-19 vaccine that targets the XBB.1.5 variant of the coronavirus. The FDA has signed off on the new protein-based shot, which joins two XBB.1.5-directed mRNA-based vaccines from Pfizer/BioNTech and Moderna as options for the upcoming immunisation campaign in the US.

BioSpace
OCTOBER 4, 2023
Following a fatality due to the rare blood disease hemophagocytic lymphohistiocytosis, the regulator slapped a partial clinical hold on two studies of Innate Pharma’s investigational therapy lacutamab.

Fierce Pharma
OCTOBER 4, 2023
After sparring with GSK’s ViiV Healthcare over the price of its HIV medication dolutegravir, Colombia appears to be taking matters into its own hands. | Colombian authorities say they plan to issue a compulsory license for ViiV Healthcare's dolutegravir, which goes by the name Tivicay on its own or as Dovato when combined with other therapeutics.

Cloudbyz
OCTOBER 4, 2023
Electronic Data Capture (EDC) systems are widely used in clinical trials to collect, manage, and analyze data from clinical studies. While EDC systems primarily focus on data capture and management, integrating trial budget management software within the EDC system can offer several advantages: 1. Streamlined Workflow: Combining EDC and budget management functionalities within the same system can create a more streamlined workflow for clinical trial teams.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content