5 FDA decisions to watch in the fourth quarter
Bio Pharma Dive
OCTOBER 2, 2023
The regulator is considering approval of what would be the first CRISPR medicine, as well as important clearances for Alnylam, Bristol Myers, Amgen and Pfizer.

Bio Pharma Dive
OCTOBER 2, 2023
The regulator is considering approval of what would be the first CRISPR medicine, as well as important clearances for Alnylam, Bristol Myers, Amgen and Pfizer.

Pharmaceutical Technology
OCTOBER 2, 2023
The Physiology and Medicine Prize has gone to two researchers whose work laid the foundation for Pfizer and Moderna’s Covid-19 vaccines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 2, 2023
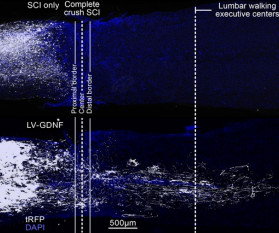
Researchers have reversed ‘irreversible paralysis’ in mice with complete spinal cord injuries using gene therapy. The team found that regrowing neurons is not enough to fully restore walking and that regenerative therapies must target specific neurons and guide them back to where they belong, which was previously unknown.
Pharmaceutical Technology
OCTOBER 2, 2023
An NDA submission for EryDex is currently intended for Q4 2025, assuming positive Phase III study results.
Bio Pharma Dive
OCTOBER 2, 2023
Silos abound in the pharma world, especially when it comes to customer care and call center support. Using AI can change everything, though. Here’s how.

Rethinking Clinical Trials
OCTOBER 2, 2023
The Patient-Centered Outcomes Core has developed a new tool kit to provide resources to support the capture of patient-reported outcome (PRO) measures in diverse study populations. The tool kit is intended for research teams conducting pragmatic clinical trials, including those participating in the NIH Pragmatic Trials Collaboratory’s Demonstration Projects.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 2, 2023
The Pharmacy Council of India (PCI) is working towards finalisation of a draft for introducing clinical pharmacists in the healthcare system for patients counselling and recording the medical history of patients, apart from other efforts including works towards rational use of medicine and safe disposal of medicine guidelines.

Bio Pharma Dive
OCTOBER 2, 2023
The rejection of lebrikizumab is Lilly’s second for an immunology drug this year, following a complete response letter for mirikizumab in April.

Pharmaceutical Technology
OCTOBER 2, 2023
The device allows patients to administer their therapy subcutaneously from home or in the clinic to increase flexibility in their daily lives.

Bio Pharma Dive
OCTOBER 2, 2023
The divestments will bring in more than $3 billion as the company works to reduce its debt and plan for future business development.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
OCTOBER 2, 2023
Many pharma companies argue that Medicare’s gross spending disregards rebates, discounts and fees paid to prescription drug insurance plans.

Bio Pharma Dive
OCTOBER 2, 2023
It’s time to adopt open-source technology – but not without security, compliance and guardrails.

Pharmaceutical Technology
OCTOBER 2, 2023
Novartis unveiled positive top-line Phase III results for iptacopan and plans talks with the FDA to obtain accelerated approval in 2024.

Rethinking Clinical Trials
OCTOBER 2, 2023
Speakers Claire Snyder, PhD Professor Johns Hopkins Schools of Medicine and Public Health Norah Crossnohere, PhD Assistant Professor Ohio State University College of Medicine Anne Schuster, PhD Research Scientist Ohio State University College of Medicine Slides Keywords Patient-Reported Outcomes, PROs, PROTEUS Consortium Key Points The Patient-Reported Outcomes Tools: Engaging Users & Stakeholders (PROTEUS) Consortium initially focused on PROs in clinical trials and then expanded

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
OCTOBER 2, 2023
The pharma company is seeking US approval of the drug for three bacterial diseases, with a PDUFA date of 3 April 2024.
Bio Pharma Dive
OCTOBER 2, 2023
De-risk your biomanufacturing- an essential mini-guide to detection and quantitation of residuals.

Pharmaceutical Technology
OCTOBER 2, 2023
Biogen’s Tofidence scores the US FDA approval for all arthritis indications similar to Roche’s Actemra (tocilizumab).

Pharma Times
OCTOBER 2, 2023
The proposed changes could limit patients’s access to treatments and clinical trials - News - PharmaTimes

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
OCTOBER 2, 2023
Novo Nordisk has joined the long list of biopharma companies that are challenging drug price negotiation provisions in the Inflation Reduction Act (IRA). | Two days after filing a lawsuit questioning the constitutionality of drug-price negotiations in the Inflation Reduction Act, Novo Nordisk has grudgingly signed on to the program. In addition, an Ohio court rejected a U.S.

Drug Patent Watch
OCTOBER 2, 2023
The FDA conducted a study to identify factors that may predict the likelihood of generic drug marketing applications. The study focused on abbreviated new drug applications (ANDAs) submitted to the… The post Factors that May Predict the Likelihood of Generic Drug Marketing Applications appeared first on DrugPatentWatch - Make Better Decisions.

Fierce Pharma
OCTOBER 2, 2023
Even as Biogen weighs strategic options for its biosimilars unit, the group is celebrating an industry first. | The exact launch timing of the biosimilar remains unclear, but Roche said it expects competition to kick off in the second half of 2023.
Pharma Times
OCTOBER 2, 2023
The study analysed and compared information from 65,000 MS patients - News - PharmaTimes

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

XTalks
OCTOBER 2, 2023
There’s an ongoing trend amongst health-conscious consumers as they carefully inspect labels and demand products with minimal ingredients. Joining the movement, Dallas-based Mooala, recently unveiled its latest innovation: the Mooala Simple line. The company claims it’s the first-ever three-ingredient, shelf-stable organic plant-based milk line.

Fierce Pharma
OCTOBER 2, 2023
Hot off an FDA approval for its Pompe disease combo treatment, Amicus Therapeutics has reeled in a major investor. | Amicus unveiled a $430 million financing pact with Blackstone Life Sciences and Blackstone Credit. The deal will see the asset manager furnish Amicus with a $400 million loan that will be used for the refinancing of existing debt, Amicus said in a press release.

BioSpace
OCTOBER 2, 2023
The Japanese pharma is voluntarily withdrawing its lung cancer drug mobocertinib, marketed as Exkivity, from U.S. and global markets after it missed the mark in a Phase III confirmatory trial.
Outsourcing Pharma
OCTOBER 2, 2023
Novartis announced today (October 2) that it is excited about positive top-line results from its study into iptacopan, a drug to treat IgA nephropathy (IgAN).

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
OCTOBER 2, 2023
Eli Lilly on Tuesday continued its buying spree with a $1.4 billion acquisition of the radiopharma company’s pipeline of clinical and preclinical radioligand therapies.

Fierce Pharma
OCTOBER 2, 2023
After laying out a goal to launch four new drugs in 2023, Eli Lilly has run into yet another regulatory snag. | The FDA has rejected Lilly’s investigational atopic dermatitis treatment lebrikizumab over findings uncovered during the inspection of a third-party contract manufacturing organization.
BioSpace
OCTOBER 2, 2023
On Monday, Syndax Pharmaceuticals announced that its menin inhibitor revumenib met the goal in a pivotal leukemia study and stopped the trial early. Their stock price still dropped on the news.

Fierce Pharma
OCTOBER 2, 2023
After allowing generic competition and slashing the price of its multidrug-resistant tuberculosis (MDR-TB) med Sirturo (bedaquiline) in low- and middle-income countries, Johnson & Johnson has t | The move follows months of pressure from advocacy groups, who called on the company to increase access to its tuberculosis med.
Advertisement
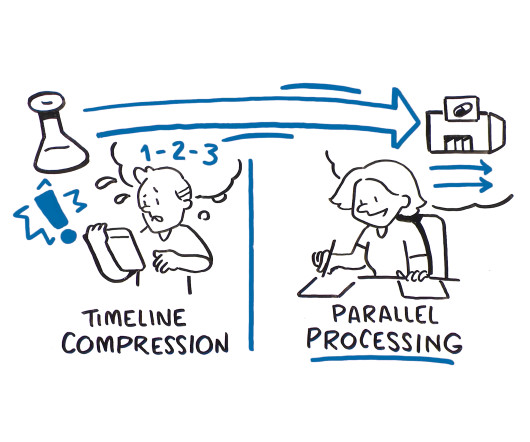
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content