SparingVision shares trial updates for rare eye disease gene therapies
Pharmaceutical Technology
SEPTEMBER 15, 2023
The company is also planning to expand its Phase I/II PRODYGY lead candidate trial in retinitis pigmentosa to three more US sites.
Pharmaceutical Technology
SEPTEMBER 15, 2023
The company is also planning to expand its Phase I/II PRODYGY lead candidate trial in retinitis pigmentosa to three more US sites.

Bio Pharma Dive
SEPTEMBER 15, 2023
The antitrust regulator is examining what it describes as abuse of the FDA “Orange Book” — another step in its scrutiny of pharma business practices.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
SEPTEMBER 15, 2023
Cure Genetics has entered a partnership and licensing deal with Frametact to develop gene therapy for familial neurological ailments.

Bio Pharma Dive
SEPTEMBER 15, 2023
Janssen will be recast as Johnson & Johnson Innovative Medicine, melding the drugs division’s identity more closely with its parent.

Pharmaceutical Technology
SEPTEMBER 15, 2023
The therapy will be available to patients who have insomnia multiple times a week, do not respond to CBTi, and whose wakeful day is severely impacted.

Fierce Pharma
SEPTEMBER 15, 2023
Questions first circulated about decongestant pills containing phenylephrine in 2007. | Several companies, including Johnson & Johnson, GSK, Procter & Gamble and Walgreens, face class-action lawsuits that claim the drugmakers knew that over-the-counter cold and flu pills containing phenylephrine did not work as advertised.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Fierce Pharma
SEPTEMBER 15, 2023
Just a few months into Bill Anderson's tenure as Bayer's CEO, the helmsman is reportedly looking to trim the drugmaker's management ranks. | The CEO is eying some big changes, but he wants to show investors he's prepared to act swiftly, Reuters reports. The publication cited three people "familiar with the matter" in reporting the behind-the-scenes developments at Bayer.
Pharmaceutical Technology
SEPTEMBER 15, 2023
Charles River has signed a multi-programme collaboration agreement with Related Sciences (RS) for the AI-powered drug platform, Logica.

Drug Discovery World
SEPTEMBER 15, 2023
Advanced Therapies Europe took place from 9-12 September 2023 in Portugal. DDW’s Megan Thomas asked attendees: What is most important to scale from discovery to commercialisation of cell and gene therapies (CGTs)? Joel Eichmann, Co-Founder, Green Elephant Biotech It always comes down to time-to-market and risk minimisation. To cover these, it is critical to choose [.
Pharmaceutical Technology
SEPTEMBER 15, 2023
The EMA CHMP has recommended granting marketing authorisation for Moderna’s updated Covid-19 vaccine targetting XBB.1.5 sublineage.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharma Times
SEPTEMBER 15, 2023
The site will contribute to the development and commercialisation of antibody drugs - News - PharmaTimes

Pharmaceutical Technology
SEPTEMBER 15, 2023
A new report found that the Remote Patient Monitoring (RPM) sector will grow, since the pandemic revealed the benefits of remote healthcare.

Fierce Pharma
SEPTEMBER 15, 2023
Lyndra Therapeutics is laying off roughly 23% of its staff, two months after the company swapped out longtime CEO Patricia Hurter, Ph.D., and as a pivotal trial for a long-acting, oral schizophreni | Lyndra Therapeutics is laying off staff two months after a CEO swap and as interim data from a pivotal trial for a schizophrenia drug nears. The company's lead asset is a long-acting, oral version of Johnson & Johnson's Uzedy.

Pharmaceutical Technology
SEPTEMBER 15, 2023
Abcam founder Jonathan Milner has declared his intention to vote against the acquisition of the company in which he holds a large stake.

Fierce Pharma
SEPTEMBER 15, 2023
With the potential for more than two dozen label expansions on the horizon, Bristol Myers Squibb bets its pipeline can help it withstand the pressure from the Inflation Reduction Act and a trio of | With the potential for more than two dozen label expansions on the horizon, Bristol Myers Squibb bets its pipeline can help it withstand the pressure from the Inflation Reduction Act and a trio of weighty patent losses.
Pharmaceutical Technology
SEPTEMBER 15, 2023
The positive opinion relied on a comparator study that showed the subcutaneous version was non-inferior to the intravenous one.

Pharma Times
SEPTEMBER 15, 2023
The method could also be beneficial for Parkinson’s disease and strokes - News - PharmaTimes
Pharmaceutical Technology
SEPTEMBER 15, 2023
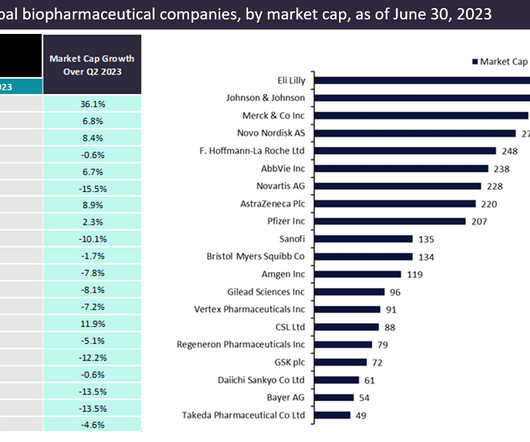
Market capitalisation increased by a total of 2.3% from $3.49tn in Q1 2023 to $3.56tn in Q2 2023, according to GlobalData.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
SEPTEMBER 15, 2023
A “surprised and extremely disappointed” PTC Therapeutics is scrambling to keep its Duchenne muscular dystrophy therapy Translarna on the European market after a detrimental review. | A “surprised and extremely disappointed” PTC Therapeutics is scrambling to keep its Duchenne muscular dystrophy therapy Translarna on the European market after a detrimental review.

Pharmaceutical Technology
SEPTEMBER 15, 2023
Generate:Biomedicines has secured $273m in Series C financing round to expedite its generative AI therapeutics portfolio.

Drug Channels
SEPTEMBER 15, 2023
Today’s guest post comes from Nicole Mayer, Senior Analyst at MMIT. Nicole summarizes MMIT’s research on payers’ views of two crucial market changes: (1) the Inflation Reduction Act of 2022’s requirement to cap insulin prices at $35 for Medicare beneficiaries, and (2) manufacturers’ reduction in list prices for certain insulin products. She also discusses MMIT’s research on such glucagon-like peptide 1 (GLP-1) drugs as Ozempic and Mounjaro.

Pharmaceutical Technology
SEPTEMBER 15, 2023
The FDA pushed lifileucel’s PDUFA date from November this year to 24 February 2024 citing resource constraints.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

pharmaphorum
SEPTEMBER 15, 2023
FTC fires shot across pharma's bows over 'improper' patents Phil.

Pharmaceutical Commerce
SEPTEMBER 15, 2023
Protective case manufacturer hires James Curleigh, as company prepares to further innovation and expand.
pharmaphorum
SEPTEMBER 15, 2023
Scientists find elusive cause of cell death in Alzheimer's Phil.

FDA Law Blog
SEPTEMBER 15, 2023
On September 18-20, Informa Connect will hold its annual #MDRPSummit in Chicago (and via livestream) to discuss the complex, ever-evolving laws and regulations in the government pricing and price reporting space. This three-day summit will feature numerous presentations, workshops and networking opportunities featuring government officials, drug pricing and reimbursement lawyers and experts and industry leaders.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
SEPTEMBER 15, 2023
Driving data & analytics transformation in life sciences Mike.
Drug Patent Watch
SEPTEMBER 15, 2023
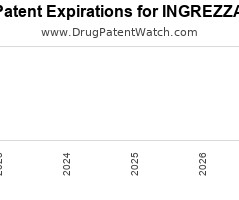
Annual Drug Patent Expirations for INGREZZA Ingrezza is a drug marketed by Neurocrine and is included in one NDA. It is available from one supplier. There are twenty patents protecting… The post New patent for Neurocrine drug INGREZZA appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
SEPTEMBER 15, 2023
FDA capacity forces delay to Iovance's cell therapy Phil.

Drug Patent Watch
SEPTEMBER 15, 2023
Annual Drug Patent Expirations for SUNOSI Sunosi is a drug marketed by Axsome Malta and is included in one NDA. It is available from two suppliers. There are thirteen patents… The post New patent for Axsome Malta drug SUNOSI appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content