The Year Ahead in Trends, Challenges, and Opportunities for Clinical Trial Sites
ACRP blog
JANUARY 10, 2024
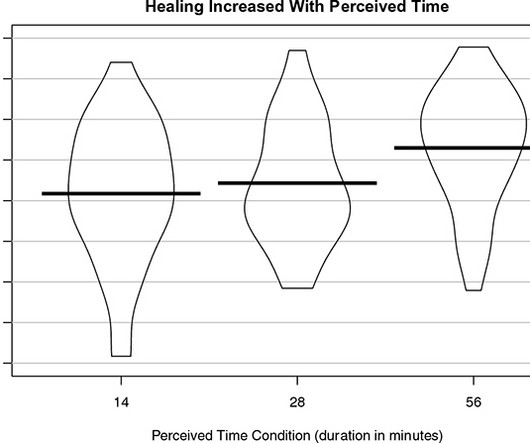
Views from ACRP Members Topics highlighted include diversity and inclusion, decentralized clinical trial (DCT) elements, workforce challenges, and a possible role for sponsors and contract research organizations (CROs) in supporting outreach by sites based in diverse communities. Nadege Gunn: Need for study-agnostic funding for sites in diverse areas “The need for improved diversity of all kinds among trial participants will continue in 2024, with sponsor and CRO support critical to success,”





































Let's personalize your content