Pfizer’s RSV vaccine wins FDA approval in older adults
Bio Pharma Dive
MAY 31, 2023
The shot’s clearance comes several weeks after the regulator made GSK’s Arexvy the first vaccine for RSV in the U.S.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Bio Pharma Dive
MAY 31, 2023
The shot’s clearance comes several weeks after the regulator made GSK’s Arexvy the first vaccine for RSV in the U.S.

Bio Pharma Dive
JULY 11, 2023
While the shot is approved in the EU, Takeda wasn’t able to address data collection issues raised by the US regulator in its current review cycle.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 1, 2021
Regulators face key decisions on COVID-19 shots for children and boosters for Moderna's and J&J's vaccines. Other closely watched drugs for multiple myeloma and depression are under review, too.

XTalks
DECEMBER 12, 2023
After receiving US Food and Drug Administration (FDA) approval for Fabhalta (iptacopan) last week for the treatment of the rare blood disorder paroxysmal nocturnal hemoglobinuria (PNH), Novartis presented trial data yesterday showing the drug’s promise in another indication.
Pharmaceutical Technology
APRIL 11, 2023
In August 2021, Zydus secured emergency use authorisation (EUA) from the Drugs Controller General of India for its Covid-19 deoxyribonucleic acid (DNA) plasmid vaccine, ZyCoV-D. The vaccine, which generates the SARS-CoV-2 viral spike protein on administration, induces the immune system’s cellular and humoral arm-mediated immune response.
pharmaphorum
AUGUST 16, 2021
Pfizer and BioNTech have formally asked for FDA approval of a third dose of their COVID-19 vaccine BNT162b2 in people aged over 16, as the US prepares to get its booster campaign underway. The post Pfizer, BioNTech file for FDA approval of COVID booster shot appeared first on.
XTalks
MAY 5, 2023
This week, the US Food and Drug Administration (FDA) approved the world’s first respiratory syncytial virus (RSV) vaccine. The shot, named Arexvy, is approved for adults aged 60 years and older and was developed by GlaxoSmithKline (GSK). Who Should Get the RSV Vaccine Arexvy?

World of DTC Marketing
SEPTEMBER 11, 2020
IN SHORT: Pharma companies have finally banded together to ask the FDA to better regulate any new drug approvals including a possible new COVID vaccine. And only about 4 in 10 would get the vaccine, even if it were free if the FDA approved it before the election. Big pharma is clearly worried.
XTalks
MAY 28, 2024
In a move signaling a major shift in influenza prevention strategies, CSL Seqirus, a global leader in flu vaccines, has transitioned from quadrivalent to trivalent formulations for the upcoming 2024/25 flu season in the US.

pharmaphorum
SEPTEMBER 16, 2021
Takeda has had a run of bad luck with its Wave1 pipeline of new drug candidates of late, but can now celebrate a vestry after getting FDA approval for first-in-class lung cancer therapy Exkivity. The post Takeda gets a win for its Wave1 pipeline, as Exkivity nabs FDA approval appeared first on.

XTalks
NOVEMBER 9, 2023
The Alinity m high risk (HR) HPV assay is approved for HPV detection and use in routine cervical cancer screening as per professional medical guidelines, explained Abbott in a press release announcing the FDA approval.

The Pharma Data
APRIL 26, 2022
Before now, Veklury was only approved to treat certain adults and pediatric patients (12 years of age and older who weigh at least 40 kilograms, which is about 88 pounds) with COVID-19. “As director of the FDA’s Center for Drug Evaluation and Research. The FDA urges the public to get vaccinated and receive a booster when eligible.
BioPharma Reporter
AUGUST 24, 2021
Full approval for the Pfizer BioNTech COVID-19 vaccine, announced by the FDA yesterday, has spurred hopes that more people will take up the vaccine.

Pharmaceutical Technology
APRIL 24, 2023
Since then, the field of nanomedicine has steadily progressed to reach high points such as the successful use of nanotechnology to deliver messenger RNA (mRNA)-based Covid-19 vaccines. In the case of most mRNA vaccines, a lipid nanoparticle-based approach was chosen due to its ability to protect the mRNA in the body and prevent degradation.

FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts. The GAO Report further explained that the agency did not have the resources to regulate the estimated 100,000 OTC drugs marketed through the monograph process.

Advarra
JANUARY 11, 2024
While mRNA usage has played several roles in clinical research , oncology researchers in particular are eager to explore the possibilities of mRNA-based cancer vaccines. The mRNA constructs used in COVID-19 vaccines, for example, direct cells to produce a version of the “spike” protein studding the surface of SARS-CoV-2.
XTalks
JUNE 2, 2021
The sotorasib approval is therefore a major breakthrough in the world of cancer and targeted therapies. The oral treatment was approved for adults with locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy.
XTalks
APRIL 21, 2021
More cases of blood clots in those vaccinated against COVID-19 — this time in six women who took the J&J shot — has US regulators recommending that vaccination efforts be paused. Read the full articles here: First Non-Surgical Heart Valve Wins FDA Approval for Severe Pulmonary Valve Regurgitation.

XTalks
SEPTEMBER 23, 2021
NYSE: PFE ) and BioNTech SE ( Nasdaq: BNTX ) announced the very first results from a pivotal trial being conducted for their COVID-19 vaccine for children on Monday. The results showed that the vaccine was safe, well-tolerated and produced robust neutralizing antibody responses in children five to 11 years old. Pfizer Inc.

pharmaphorum
FEBRUARY 11, 2021
AstraZeneca is aiming to get a next-generation vaccine targeting emerging variants approved and into the arms of patients by the autumn, company representatives said in a press conference. While the vaccine is approved in Europe, the UK and several other countries, FDA approval hangs on a study in the US.

XTalks
APRIL 26, 2021
After years of disappointing malaria vaccine trials, a malaria shot developed by researchers at the Jenner Institute at the University of Oxford has demonstrated an unprecedentedly high efficacy of 77 percent, and may be the magic bullet the world has been waiting for against the deadly disease.

pharmaphorum
SEPTEMBER 15, 2022
Pfizer’s close-fought contest with GSK to bring a next-generation meningitis vaccine to market has entered the final rounds, with the former ahead on points following the readout of a pivotal phase 3 clinical trial. None of them had been previously vaccinated against MenB. Pfizer doesn’t break out sales of Trumenba.

The Pharma Data
MAY 7, 2021
The Pfizer-BioNTech COVID-19 Vaccine is currently available in the U.S. under an Emergency Use Authorization (EUA) granted by the FDA on December 11, 2020. Since then, the companies have delivered more than 170 million doses of the vaccine across the U.S. CEO and Co-founder of BioNTech. “We We are pleased to work with U.S.

The Pharma Data
NOVEMBER 21, 2021
People with achondroplasia have a genetic mutation that causes a certain growth regulation gene called fibroblast growth factor receptor 3 to be overly active, which prevents normal bone growth. The most common side effects of Voxzogo include injection site reactions, vomiting and decreased blood pressure. Source link: [link].

The Pharma Data
NOVEMBER 29, 2021
National Cancer Institute: Ovarian, Fallopian Tube, and Primary Peritoneal Cancer FDA Approved Drugs: Questions and Answers. ###. The FDA, an agency within the U.S. Source link: [link].

The Pharma Data
NOVEMBER 12, 2021
Treatment is First FDA-Approved Option Patients Can Take Regardless of Previous Therapies. Food and Drug Administration approved Besremi (ropeginterferon alfa-2b-njft) injection to treat adults with polycythemia vera, a blood disease that causes the overproduction of red blood cells. Today, the U.S. Source link: [link].

The Pharma Data
MAY 4, 2021
Pfizer plans to file for full FDA approval of Covid vaccine at the end of this month ( CNBC ). The FDA is set to authorize the Pfizer-BioNTech vaccine for those 12-15 years old by early next week. White House to shift COVID-19 vaccine to states with more need ( Reuters ).

The Pharma Data
MARCH 29, 2022
Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series.

pharmaphorum
APRIL 6, 2021
AstraZeneca is shifting production of its COVID-19 vaccine away from a plant in Baltimore which also makes Johnson & Johnson’s shot, after human error resulted in the contamination of 15 million doses. . Emergent claims however that one batch of the vaccine simply failed quality controls, and no contamination took place.

XTalks
FEBRUARY 3, 2021
COVID-19 vaccines have been a topic of conversation since the outbreak began, and last year’s US Food and Drug Administration (FDA) approval of the Pfizer/BioNTech and Moderna vaccines have given the world hope to defeat the COVID-19 pandemic. One country that has already vaccinated 1.8

The Pharma Data
MAY 7, 2021
. Pfizer-BioNTech files for US approval of COVID-19 vaccine ( Reuters ) ( NYTimes ) ( Politico ) ( Press ). Covid-19 Vaccines Are Wasted as Special Syringes Run Short ( WSJ ). Adcomm splits slightly in favor of FDA approving ChemoCentryx’s rare disease drug ( Endpoints ).

The Pharma Data
MAY 13, 2021
CDC Advisers Endorse Pfizer Vaccine for Children Ages 12 to 15 ( NYTimes ) ( Reuters ) ( STAT ). US CDC finds more clotting cases after J&J vaccine, sees causal link ( Reuters ) ( NBC ) ( NYTimes ). Covid vaccines may not protect people with immune disorders. In Focus: US.

The Pharma Data
FEBRUARY 24, 2022
Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. Source link: [link].
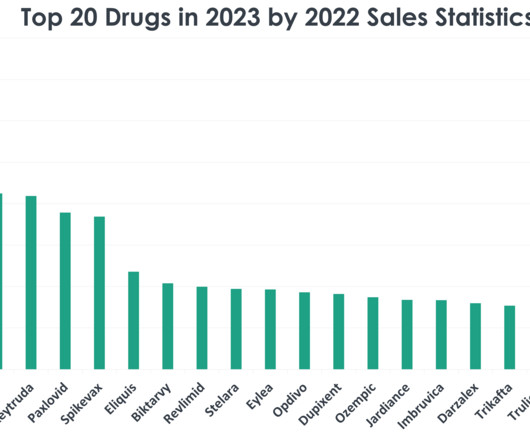
XTalks
DECEMBER 19, 2023
1) Comirnaty (COVID-19 Vaccine, mRNA) Sales in 2022: $37.81 billion Company: Pfizer Disease it is FDA-approved to treat: Comirnaty is an active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older. The rise of the Omicron variant fuelled the sale of the vaccine.

The Pharma Data
MARCH 15, 2022
Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. Source link: [link] ov/.

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been recommended for conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been granted a conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.

The Pharma Data
NOVEMBER 20, 2020
Food and Drug Administration has scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Dec. 10 to discuss the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, Inc. Source: FDA. BNT162b2 (SARS-CoV-2 vaccine) FDA Approval History.

The Pharma Data
JANUARY 20, 2021
FDA Approves Verquvo (vericiguat) for Heart Failure with Reduced Ejection Fraction. When NO binds to sGC, the enzyme catalyzes the synthesis of intracellular cyclic guanosine monophosphate (cGMP), a second messenger that plays a role in the regulation of vascular tone, cardiac contractility, and cardiac remodeling.

The Pharma Data
DECEMBER 30, 2020
30 December 2020 — AstraZeneca’s COVID-19 vaccine has been approved for emergency supply in the UK, with the first doses being released today so that vaccinations may begin early in the New Year. This is the first authorisation for this vaccine.

The Pharma Data
NOVEMBER 23, 2021
Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of mortal and veterinary medicines, vaccines and other natural products for mortal use, and medical bias. Source link: [link].

The Pharma Data
JANUARY 15, 2021
Food and Drug Administration (FDA) approval of Darzalex Faspro ® (daratumumab and hyaluronidase-fihj), a subcutaneous formulation of daratumumab, in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd) for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis.[1] Janssen Biotech, Inc.

The Pharma Data
DECEMBER 18, 2020
Food and Drug Administration issued an emergency use authorization (EUA) for the second vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The emergency use authorization allows the Moderna COVID-19 Vaccine to be distributed in the U.S. Hahn , M.D.

The Pharma Data
JANUARY 6, 2021
6 January 2021 — AstraZeneca’s COVID-19 vaccine has been granted emergency use authorisation in India as well as Argentina, Dominican Republic, El Salvador, Mexico and Morocco for the active immunisation of adults. Additional safety and efficacy data will continue to accumulate from ongoing clinical trials.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content