Innovation and development of generic pharmaceuticals
pharmaphorum
MAY 18, 2023
Innovation and development of generic pharmaceuticals Mike.Hammerton Thu, 05/18/2023 - 10:17 Bookmark this
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 Generic Pharmaceutical Related Topics
Generic Pharmaceutical Related Topics 
pharmaphorum
MAY 18, 2023
Innovation and development of generic pharmaceuticals Mike.Hammerton Thu, 05/18/2023 - 10:17 Bookmark this

BioTech 365
SEPTEMBER 17, 2020
DUBLIN–(BUSINESS WIRE)–The “Global Generic Pharmaceutical Partnering Terms and Agreements 2010-2020” report has been added to ResearchAndMarkets.com’s offering.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JUNE 1, 2023
Dipharma is a pioneer in developing improved generic pharmaceutical products for rare diseases: our desire to innovate and our engagement do not stop, but every day we continue to seek new and better solutions for patients around the world.”

Pharmaceutical Technology
FEBRUARY 28, 2023
Novartis overview Novartis is a healthcare company that focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical products and eye care products.

Drug Patent Watch
JULY 25, 2024
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2019 to 2024. Companies that successfully challenge patents on branded drugs are granted…

Pharmaceutical Technology
FEBRUARY 28, 2023
Novartis overview Novartis is a healthcare company that focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical products and eye care products.

BioTech 365
AUGUST 4, 2021
the “Company”) (OTC PINK:SHMN), a generic Pharmaceutical, Nutraceutical, and Cosmeceutical company that manufactures and markets generic … Continue reading → Chino Hills, 08/04/2021 / 15:52, CET/CEST – EQS Newswire – Sohm, Inc. CHINO HILLS, CA / ACCESSWIRE / August 4, 2021 / SOHM, Inc.

BioTech 365
MAY 18, 2021
OTC PINK:SHMN), a generic pharmaceutical, nutraceutical, and cosmeceutical company that manufactures and markets generic drugs covering … Continue reading → Chino Hills, 05/18/2021 / 15:31, CET/CEST – EQS Newswire – Sohm, Inc. CHINO HILLS, CA / ACCESSWIRE / May 18, 2021 / SOHM, Inc.

BioTech 365
JUNE 3, 2021
the “Company”) (OTC PINK:SHMN), generic Pharmaceutical, Nutraceutical, and Cosmeceutical company that manufactures and markets generic drugs … Continue reading → Chino Hills, 06/03/2021 / 18:51, CET/CEST – EQS Newswire – Sohm, Inc. CHINO HILLS, CA / ACCESSWIRE / June 3, 2021 / SOHM, Inc.

BioTech 365
MAY 13, 2021
First Nation Group Announces Launch of Pharmaceuticals Division First Nation Group Announces Launch of Pharmaceuticals Division First Nation Group launches new division, First Nation Pharmaceuticals, offering generic pharmaceutical distribution and supply chain management NICEVILLE, Fla.–(BUSINESS

BioTech 365
MARCH 12, 2021
Amring Pharmaceuticals Inc. –(BUSINESS WIRE)–Amring Pharmaceuticals Inc. Amring), a niche brand and generic pharmaceutical company, announced today an … Continue reading → Enters into License and Development Agreement with Amzell B.V. BERWYN, Pa.–(BUSINESS

BioTech 365
DECEMBER 31, 2020
NASDAQ: TLGT), a New Jersey-based specialty generic pharmaceutical company, today announced its financial results for the … Continue reading → Announces Third Quarter 2020 Results Teligent, Inc. Announces Third Quarter 2020 Results BUENA, N.J., 31, 2020 (GLOBE NEWSWIRE) — Teligent, Inc.
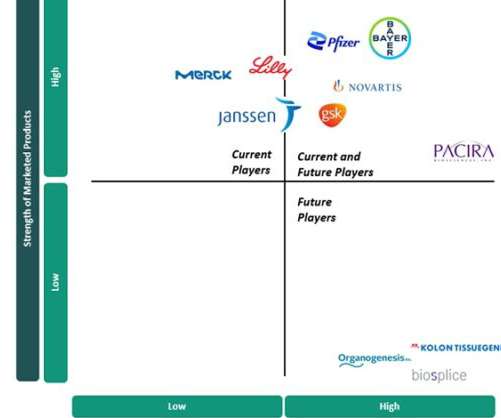
Pharmaceutical Technology
JULY 28, 2022
The current standards of care (SOCs) in OA focus on symptom management and are made up of generic pharmaceuticals, including nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, antidepressants and intra-articular (IA) injections. There are no disease-modifying drugs currently approved for OA.

Pharmaceutical Technology
FEBRUARY 28, 2023
Novartis overview Novartis is a healthcare company that focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical products and eye care products. It was also under development for the treatment of idiopathic/primary membranous nephropathy.

Pharmaceutical Technology
FEBRUARY 28, 2023
Novartis overview Novartis is a healthcare company that focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical products and eye care products. It was also under development for the treatment of idiopathic/primary membranous nephropathy.

BioTech 365
DECEMBER 2, 2020
Amring Pharmaceuticals Inc. Acquires LYSTEDA® Amring Pharmaceuticals Inc. –(BUSINESS WIRE)–Amring Pharmaceuticals Inc. Amring), a generic pharmaceutical company, announced entering into an agreement with Ferring International Center S.A. Acquires LYSTEDA® BERWYN, Pa.–(BUSINESS

BioTech 365
SEPTEMBER 23, 2020
NASDAQ: TLGT) (“Teligent” or the “Company”), a New Jersey-based specialty generic pharmaceutical company, announced the closing of the issuance of approximately $27.5 BUENA, N.J., 23, 2020 (GLOBE NEWSWIRE) — Teligent, Inc.

Drug Channels
SEPTEMBER 10, 2020
Today’s guest post comes from George Keefe, Senior Vice President of External Affairs and Public Policy at Teva Pharmaceuticals. George examines the economic impact of generic pharmaceuticals on the U.S. He argues that the generics industry provides safe, effective, and affordable medicines for many Americans.

XTalks
DECEMBER 14, 2020
AvKARE, a generics pharmaceutical manufacturer based in Pulaski, Tennessee, has issued a recall on one lot each of its sildenafil 100 mg tablets and trazodone 100 mg tablets due to a packaging “mix-up” in which both drugs were filled into the same bottle. According to AvKARE, the error occurred at a third-party bottle filling facility.
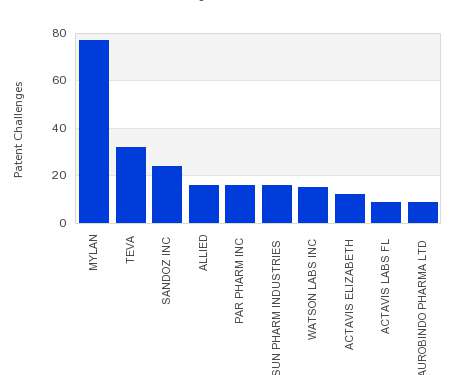
Drug Patent Watch
JULY 26, 2022
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2017 to 2022. Companies that successfully challenge patents on branded drugs are granted six…. The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
FEBRUARY 18, 2023
Novartis Overview Novartis is a healthcare company that focuses on the discovery, development, manufacture and marketing of prescription and generic pharmaceutical products and eye care products. It was under development for renal cell carcinoma as first line therapy.
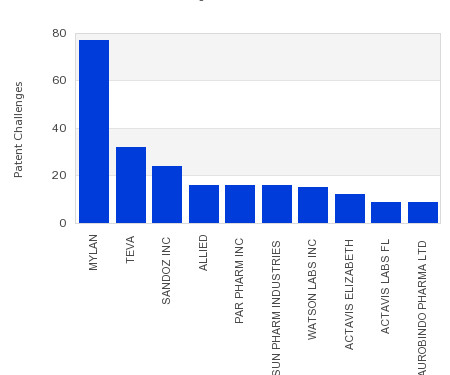
Drug Patent Watch
NOVEMBER 9, 2023
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2018 to 2023. Companies that successfully challenge patents on branded drugs are granted six… The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.
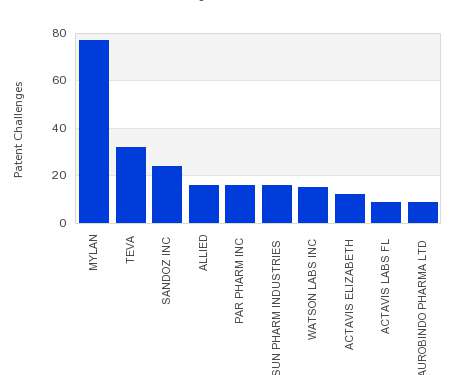
Drug Patent Watch
NOVEMBER 9, 2021
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2016 to 2021. Companies that successfully challenge patents on branded drugs are granted six…. The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
JANUARY 4, 2021
Meiji Seika Pharma, since it launched penicillin in Japan in 1946, has been providing high-quality pharmaceutical products such as antibiotics, antidepressants and antipsychotics in Japan and overseas. Integrated Reports: [link].
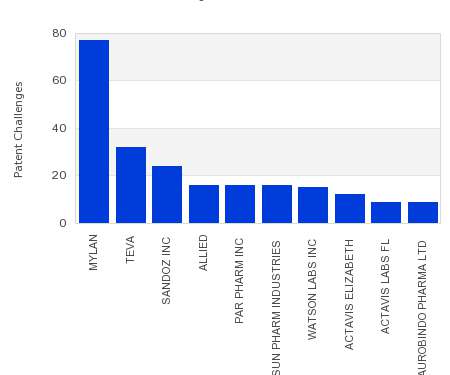
Drug Patent Watch
JULY 26, 2021
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2016 to 2021. Companies that successfully challenge patents on branded drugs are granted six…. The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MARCH 22, 2023
1 About Sandoz Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. In some cases of autoimmune disease, the immune system damages the body’s own tissues.

XTalks
DECEMBER 14, 2020
AvKARE, a generics pharmaceutical manufacturer based in Pulaski, Tennessee, has issued a recall on one lot each of its sildenafil 100 mg tablets and trazodone 100 mg tablets due to a packaging “mix-up” in which both drugs were filled into the same bottle. According to AvKARE, the error occurred at a third-party bottle filling facility.
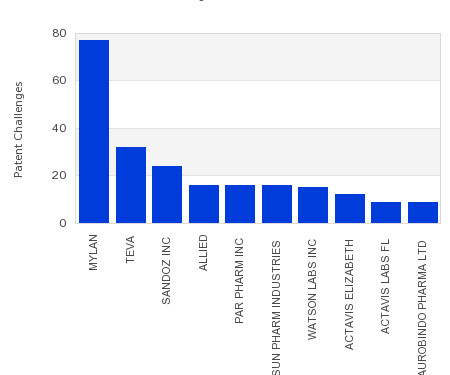
Drug Patent Watch
JULY 26, 2023
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2018 to 2023. Companies that successfully challenge patents on branded drugs are granted six… The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
NOVEMBER 5, 2020
About Jubilant Cadista Pharmaceuticals Inc. Jubilant Cadista Pharmaceuticals Inc. is a rapidly growing generic pharmaceutical company in the United States devoted to discovery, manufacture, sale, and distribution of prescription generic pharmaceutical products.

The Pharma Data
OCTOBER 27, 2020
Lannett Company, founded in 1942, develops, manufactures, packages, markets and distributes generic pharmaceutical products for a wide range of medical indications. A playback of the call will be archived and accessible on the same website for at least three months. About Lannett Company, Inc. .
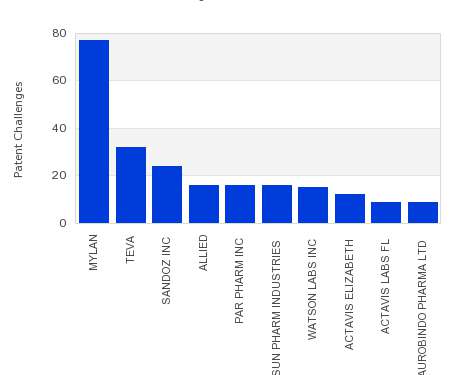
Drug Patent Watch
NOVEMBER 9, 2022
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2017 to 2022. Companies that successfully challenge patents on branded drugs are granted six…. The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
JUNE 17, 2022
About Sandoz Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. The adalimumab reference medicine (Humira ®* ) was first approved with an adalimumab concentration of 50 mg/mL.

Pharma in Brief
SEPTEMBER 19, 2023
The Plaintiffs (Boehringer companies) allege that the Defendants (generic pharmaceutical companies) would infringe six Canadian patents if allowed to market generic empagliflozin products. In defence, the Defendants allege that the patents are invalid.

The Pharma Data
APRIL 4, 2023
About Sandoz Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. The adalimumab reference medicine (Humira ® *) was first approved with an adalimumab concentration of 50 mg/mL.

XTalks
JUNE 8, 2022
Sandoz is a pioneering leader in generic pharmaceuticals and biosimilars, as Sandoz launched the world’s first biosimilar in Europe in 2006 and won the first biosimilar approval in the US in 2014. Related: Cyltezo Becomes First FDA-Approved Interchangeable Humira Biosimilar.

Drug Patent Watch
NOVEMBER 8, 2020
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2015 to 2020. Companies that successfully challenge patents on branded drugs are granted six…. The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MAY 31, 2022
About Sandoz Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. Our purpose is to pioneer access for patients by developing and commercializing novel, affordable approaches that address unmet medical needs.

The Pharma Data
MARCH 15, 2022
The FDA regularly takes steps to help guide industry through the development process for generic drug products, including combination products, such as MDIs, that consist of a drug and a device.

Pharmaceutical Technology
DECEMBER 22, 2022
The organisation is a non-profit generic pharmaceutical company and has launched its Insulin Initiative, which aims to drive affordability and accessibility for all Americans, regardless of insurance status.
The Pharma Data
MAY 8, 2021
Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. About Sandoz. Our purpose is to pioneer access for patients by developing and commercializing novel, affordable approaches that address unmet medical needs.

The Pharma Data
OCTOBER 26, 2021
Sandoz is a global leader in generic pharmaceuticals and biosimilars. Its global portfolio covers all major therapeutic areas with a global market leadership position in biosimilars, generic antibiotics and oncology medicines.

Pharma in Brief
DECEMBER 3, 2023
The Regulations became a moving target for the innovative and generic pharmaceutical industries, being amended seven times between 1998 and 2016. This was often the innovator’s only recourse as innovators were typically unable to appeal unfavourable judgments under the Regulations. Frequent amendments (1998–2016).

The Pharma Data
MAY 18, 2021
Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. About Sandoz. Our purpose is to pioneer access for patients by developing and commercializing novel, affordable approaches that address unmet medical needs.

The Pharma Data
MAY 2, 2021
Sandoz, a Novartis division, is a global leader in generic pharmaceuticals and biosimilars. Our ambition is to be the world’s leading and most valued generics company. About Sandoz. Our purpose is to pioneer access for patients by developing and commercializing novel, affordable approaches that address unmet medical needs.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content