mRNA Drugs: Pros, Cons, and Prospects
Pharma Mirror
OCTOBER 16, 2023
The first mRNA drug (BNT162b2 vaccine) was granted emergency use authorization by the FDA in December 2020 and approved for marketing in August 2021.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharma Mirror
OCTOBER 16, 2023
The first mRNA drug (BNT162b2 vaccine) was granted emergency use authorization by the FDA in December 2020 and approved for marketing in August 2021.
Pharmaceutical Technology
MAY 29, 2023
Novavax has received positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) for full marketing authorisation (MA) of its Covid vaccine, Nuvaxovid (NVX-CoV2373) , in the European Union (EU). The safety and efficacy of Nuvaxovid was evaluated as a primary series.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
APRIL 11, 2023
The approval enables the company to manufacture and market azithromycin to treat infections such as bronchitis, pneumonia and sexually transmitted diseases (STDs). The vaccine, which generates the SARS-CoV-2 viral spike protein on administration, induces the immune system’s cellular and humoral arm-mediated immune response.
BioPharma Reporter
DECEMBER 11, 2020
Sanofi and GSK announced a delay on Friday in their adjuvanted recombinant protein-based COVID-19 vaccine program to improve immune response in the elderly, saying clinical trials showed an insufficient immune response in older people.

Pharmaceutical Technology
MARCH 31, 2023
Available to people ages 16 years and above who have been vaccinated with a Covid-19 mRNA vaccine, EMA’s Human Medicines Committee concluded the vaccine is ready for marketing authorization in the EU, on 30 March. Bimervax is a recombinant protein subunit vaccine, marketed by the Girona, Spain-based Hipra.
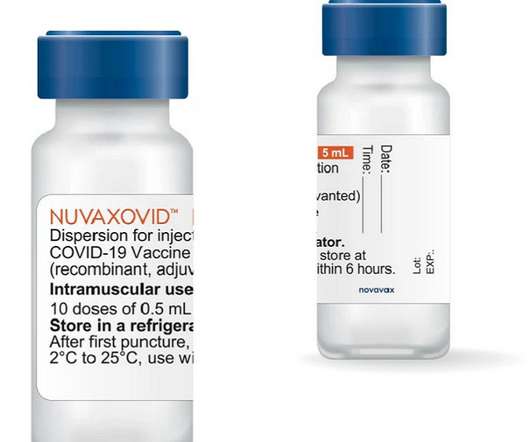
Pharmaceutical Technology
JANUARY 19, 2023
Nuvaxovid is a protein-based vaccine created from the genetic sequence of the initial SARS-CoV-2 virus strain. The expanded manufacturing and marketing approval was based on the data obtained from the Phase II trial, conducted in Australia and the US; a separate Phase II trial held in South Africa; and the UK-sponsored COV-BOOST trial.

The Pharma Data
JUNE 29, 2021
A sub-analysis from the Oxford-led COV001 and COV002 trials with Vaxzevria induced strong immune responses following either a prolonged second dose interval of up to 45 weeks or following a third boosting dose. There is an excellent response to a second dose, even after a 10 month delay from the first.”.

World of DTC Marketing
DECEMBER 29, 2020
Once introduced into the human body, the protein-making machinery uses this genetic material in our cells to churn out the coronavirus “spike protein,” triggering an immune response. It’s estimated that the COVID 19 vaccine market could be worth almost $100 billion. That’s a hell of an incentive.
pharmaphorum
JANUARY 5, 2022
A COVID-19 vaccine that could work against multiple variants of the coronavirus – developed by US biotech Gritstone bio – has generated encouraging immune response data in its first clinical trial. “With new variants – like Omicron and B.1.640.2

Pfizer
JULY 26, 2022
Pfizer and BioNTech Advance COVID-19 Vaccine Strategy With Study Start of Next-Generation Vaccine Candidate Based on Enhanced Spike Protein Design. Pfizer and BioNTech Advance COVID-19 Vaccine Strategy With Study Start of Next-Generation Vaccine Candidate Based on Enhanced Spike Protein Design. deliesschef. Tue, 07/26/2022 - 17:41.

pharmaphorum
DECEMBER 4, 2020
Under the agreement Valneva could receive up to $308 million in cash payments, after Pfizer paid $130 million up front for marketing rights to the vaccine if it succeeds in the clinic. OspA is one of the most dominant surface proteins expressed by the bacteria when present in the ticks that spread the disease.

The Pharma Data
APRIL 15, 2022
BioNTech is the Marketing Authorization Holder in the United States, the European Union, the United Kingdom, Canada and the holder of emergency use authorizations or equivalents in the United States (jointly with Pfizer) and other countries.

Drug Discovery World
NOVEMBER 30, 2022
Associate Professor Keith Chappell, co-leader of UQ’s Rapid Response Vaccine Pipeline, said pre-clinical testing had shown the ‘Clamp2’ platform was meeting all expectations, producing stabilised antigens and inducing strong neutralising immune responses. “We Clamp technology .

pharmaphorum
SEPTEMBER 30, 2020
This first data comes from a descriptive analysis from the seamless phase 1/2/3 trial that Regeneron hopes will hurry the medicine dubbed REGN-COV2 to market. As expected, patients in the study formed two populations: those who had already mounted an immune response and those whose immune response was not yet adequate.
pharmaphorum
FEBRUARY 22, 2021
In December interim phase 1/2 trial results showed the vaccine produced a lower immune response in older adults. At the time analysts said that this was likely due to an insufficient concentration of the antigen and the companies have refined their antigen formulation hoping for a better immune response including in older adults.

Advarra
JANUARY 11, 2024
Adoptive T Cell therapies, therapeutic antibodies, and immunomodulatory proteins represent just some of the potentially beneficial treatment strategies for successful mRNA cancer trials. The mRNA constructs used in COVID-19 vaccines, for example, direct cells to produce a version of the “spike” protein studding the surface of SARS-CoV-2.

pharmaphorum
JULY 14, 2021
The shot also includes two “conserved” pneumococcal proteins – antigens that seem always to be present in the bacterium regardless of its serotype, so could potentially provide protection against an even broader range.

XTalks
JUNE 5, 2023
Abrysvo is an unadjuvanted vaccine and is composed of two preF proteins selected to optimize protection against RSV A and B strains. “A In February 2023, Pfizer revealed that the European Medicines Agency (EMA) has accepted the Marketing Authorization Application (MAA) for Abrysvo. billion in value by 2024, and grow to $9.53

Drug Discovery World
APRIL 11, 2024
We have noted that cancer patients can develop powerful anti-tumour immune responses that can result in complete cure of the disease and we call these patients ‘elite responders’. By understanding what triggers the adaptive immune response in “elite responders” we have identified new targets and therapeutic antibodies.

Cloudbyz
MARCH 5, 2024
Biologics: Biologics are large, complex molecules, often proteins, that are produced using living cells. Mode of Action : Small Molecules: They often work by binding to specific sites on target proteins to inhibit or activate their function, which can affect various biological pathways inside the cell.

pharmaphorum
NOVEMBER 23, 2021
The two had been working on a COVID-19 vaccine, which showed promise in early-stage clinical testing, but shelved the project in September after deciding it would be too late to a market already dominated by rival shots from Pfizer/BioNTech and Moderna. Specific financial terms have not been disclosed.

Drug Discovery World
NOVEMBER 13, 2023
Ahead of protein and antibody engineering conference PEGS Europe 2024 in Lisbon, DDW’s Megan Thomas looks at what to expect from each track of the annual biologics technology meeting. Ali Madani, PhD, Founder and CEO, Profluent Bio, on: ‘Protein engineering with large language models’.

Cloudbyz
MARCH 6, 2024
Understanding Biologics: Biologics are a class of therapeutic agents derived from living organisms, such as cells, tissues, or proteins. Personalized Medicine: Biologics offer the potential for personalized treatment approaches by taking into account individual variations in genetics, immune responses, and disease characteristics.

Drug Discovery World
MAY 11, 2023
Kerstin Pohl , Senior Global Marketing Manager, Gene Therapy & Nucleic Acid at SCIEX, looks at the application of liquid chromatography-mass spectrometry for analysing host cell proteins. Host cell proteins (HCPs) – proteins derived from the host cells used for viral production—are one class of process-related impurities.

Pharmaceutical Technology
JULY 29, 2022
Once new vaccines for the Omicron variant are approved, they will partly make up for the waning interest in older Covid boosters in developed markets. Bivalent vaccines work by stimulating an immune response against two different antigens, whereas monovalent vaccines only target one antigen. Commercial dose manufacturing.
pharmaphorum
MAY 31, 2022
billion takeover of US biotech Affinivax, buying a pneumococcal vaccine candidate that is aiming to break into a market that for years has been dominated by Pfizer’s Prevnar franchise. The biotech says this can stimulate both B cell (antibody) and T cell immune responses with a single shot. GlaxoSmithKline has agreed a $3.3
Drug Discovery World
JULY 5, 2023
Global Cancer Segment Market Manager at Agilent Technologies, recently sat down with DDW Editor Reece Armstrong to tell us about the developments in the ‘golden age’ of cancer research. MG: I am responsible for Agilent’s cancer segment. Mark Garner, PhD., RA: Please can you tell us a bit more about your role at Agilent?

Delveinsight
SEPTEMBER 8, 2021
As per DelveInsight’s report on Benlysta (Belimumab) market forecast , the sale of Belimumab has kept on increasing year after year. Currently, the SLE therapeutics market is revolving around only one drug i.e It is the top-selling medication in the market. Anifrolumab (MEDI-546). Baricitinib (INCB-028050).
pharmaphorum
JULY 20, 2021
The vaccine – now dubbed Vidprevtyn – is based on a recombinant protein antigen developed by Sanofi’s vaccines unit Sanofi Pasteur, and also includes an immune-boosting adjuvant developed by GSK that aims to boost its efficacy. 1.351) variant first identified in South Africa.

World of DTC Marketing
APRIL 11, 2021
Based on the virus a number of vaccines targeting the spike protein were designed, tested in animal models and found to be quite promising against SARS and other coronavirus illnesses like Middle East respiratory syndrome. However, the continued study of the virus was shelved because of the lack of funding.

The Pharma Data
JANUARY 21, 2021
Luckily, the new variants still rely on the coronavirus’ “spike protein” to infect cells, and the two COVID vaccines now on the U.S. market specifically target the spike protein to prevent transmission, explained Dr. Kathryn Edwards, scientific director of the Vanderbilt University Vaccine Research Program in Nashville.
pharmaphorum
MAY 27, 2021
GSK and Sanofi think they could be on track for approval of the recombinant protein-based vaccine – delivered with GSK’s immune-response boosting adjuvant – before the end of the year. The phase 3 trial will include one shot targeting the original SARS-CoV-2 virus as well as a second against the B.1.351

pharmaphorum
MARCH 17, 2022
.” Both Pfizer’s BioNTech-partnered Comirnaty and Moderna’s SpikeVax vaccines use LNPs to carry mRNA coding for the SARS-CoV-2 spike protein. After dosing, the LNPs deliver the mRNA sequence into cells, which is then used by cellular machinery to manufacture the viral protein and stimulate an immune response.
pharmaphorum
AUGUST 23, 2021
DNA vaccines work by delivering a genetically-engineered plasmid containing the DNA sequence encoding the desired antigen – in this case the SARS-CoV-2 spike protein – which is then taken up by cells. Proponents of the approach claim that DNA vaccines may have advantages over other technologies like mRNA.

Pharmaceutical Technology
AUGUST 24, 2022
More than three decades ago, scientists at the National Cancer Institute began exploring ways to fight cancer using the patient’s immune system. The global immunotherapy market, which includes CAR T-cell therapy, adoptive cell therapies, checkpoint inhibitors, monoclonal antibodies, and other immunotherapies, is expected to grow by 10.1%

XTalks
OCTOBER 25, 2022
Imjudo is an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) monoclonal antibody that blocks the action of CTLA-4. Inhibition of the protein aids in T-cell activation to prime the immune system against cancer cells to induce their death.

XTalks
MAY 1, 2023
Qalsody contains tofersen, an antisense oligonucleotide that specifically targets the mRNA made from mutated SODI genes to prevent the formation of toxic SOD1 proteins. Reduction in these two biomarkers in response to tofersen is believed to predict clinical benefit for patients. How Does Qalsody Work?

The Pharma Data
SEPTEMBER 30, 2021
The function of the adjuvant is to market the body’s immune reaction. Our results show that the candidate vaccine formulation is safe, produces rapid immune responses – within seven days – and elicits comprehensive immunity against SARS-CoV-2,” said Varadarajan.

Drug Discovery World
AUGUST 1, 2023
1 August was first chosen as World RNA Day in 2018 as a play on AUG (adenine, uracil and guanine), a triple sequence of RNA (called a codon) that initiates protein synthesis by the cell. Since then, it has been observed to publicise the importance of this molecule in the generation of proteins in the body.

pharmaphorum
SEPTEMBER 8, 2021
Kadmon claimed approval for Rezurock (belumosudil) in July as a treatment for chronic graft versus host disease (GVHD) – a common and often fatal complication that can follow a bone marrow transplant and which occurs when the donated cells mount an immune response against the transplant recipient’s tissues and organs.

The Pharma Data
MAY 27, 2022
The authorisation is based on a review by the Committee for Medicinal Products for Human Use (CHMP) of the substantial body of evidence demonstrating an increased immune response after a third dose booster with Vaxzevria following a primary vaccine schedule of either Vaxzevria or an mRNA COVID-19 vaccine.(1-5).

Drug Discovery World
NOVEMBER 8, 2023
Ahead of protein and antibody engineering conference PEGS Europe 2024 in Lisbon, DDW’s Megan Thomas looks at what to expect from each track of the annual biologics technology meeting. Marketing Product Manager, Product, NanoTemper Technologies.

XTalks
APRIL 7, 2021
The new, revised EUA will allow the company to market the test for the qualitative detection of SARS-CoV-2 from nasal swab samples for screening use with serial testing. The test is intended for the detection of COVID-19 infection, either a recent or previous infection, as detected through an adaptive antibody immune response to SARS-CoV-2.

Drug Discovery World
JANUARY 22, 2024
Entact Bio is using a platform to develop enhancement-targeting chimeric (ENTAC) molecules, which bring together target proteins with enzymes called deubiquitinating enzymes (DUBs). These include examples such as investor confidence and lower costs for the developers, as well as quicker drugs to market and ultimately, better patient care.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content