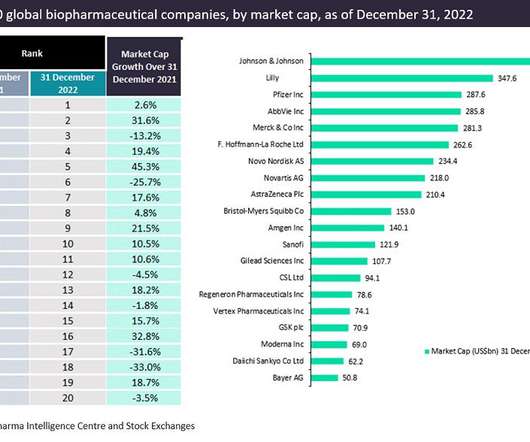
Dyadic expands Covid-19 licence with Rubic to bolster vaccine production in Africa
Pharmaceutical Technology
APRIL 13, 2023
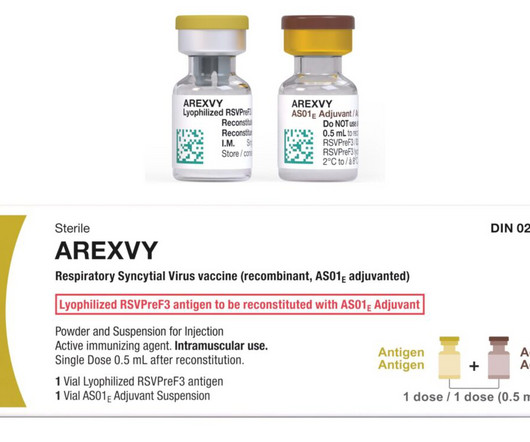
The expanded licence will include the development of vaccines and therapeutic proteins beyond Covid-19 for human and animal health markets in Africa. During the Covid-19 pandemic, vaccination rates of many countries in Africa were significantly trailing the rest of the world.

































Let's personalize your content