European regulator concludes no suicide link to obesity drugs
Bio Pharma Dive
APRIL 12, 2024
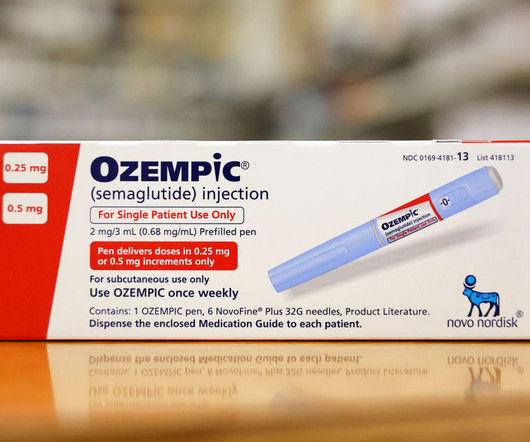
The decision clears a concern hanging over GLP-1 medicines like Ozempic since reports of suicidal ideation and self-harm among people taking the drugs surfaced in July.











































Let's personalize your content