Clinical Trial Efficiency: Top Risk Indicators and Solutions for Site Performance
Advarra
JANUARY 18, 2024
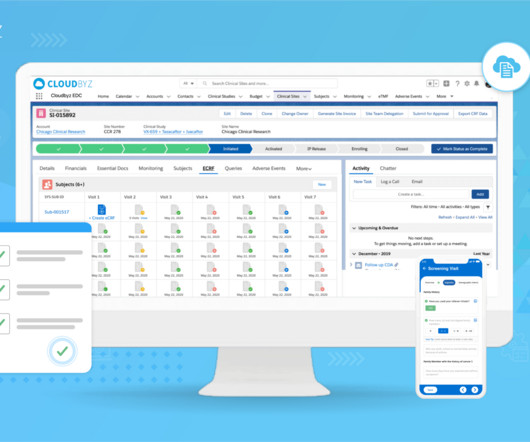
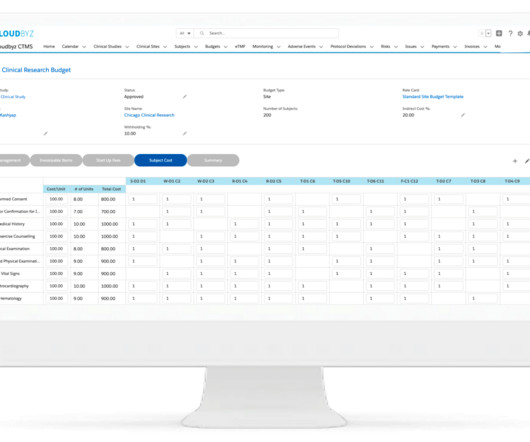
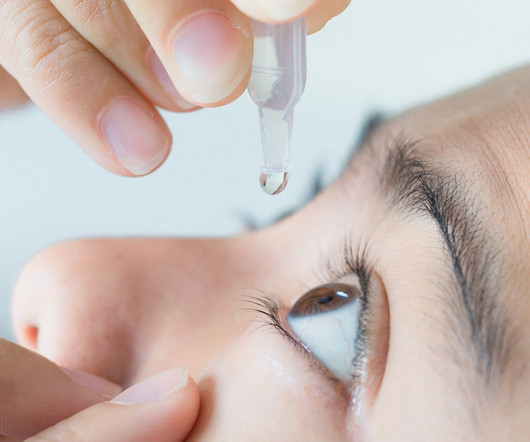
Sponsor and contract research organization (CRO) team members such as clinical research associates (CRAs), study managers, and startup specialists collaborate with research site teams to activate and execute clinical trials. Key Risk Indicator #1: Have Site Staff Accessed Your Training System and Materials?















































Let's personalize your content