Using single-cell technology to create human antibody-based therapeutics
Drug Discovery World
FEBRUARY 6, 2023
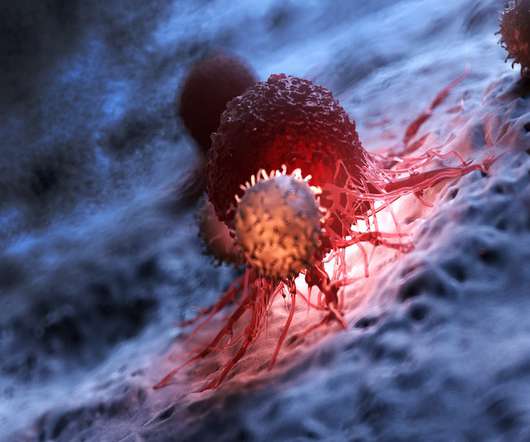
Taking a human-first approach, Infinimmune is leveraging single-cell technology to create truly human antibody-based therapeutics directly derived from the human immune system. MT: Can you provide me with an overview of what Infinimmune does currently and the potential it sees in the antibody-based therapeutics market?





























Let's personalize your content