Umoja and IASO partner to develop therapies for haematological malignancies
Pharmaceutical Technology
NOVEMBER 22, 2022
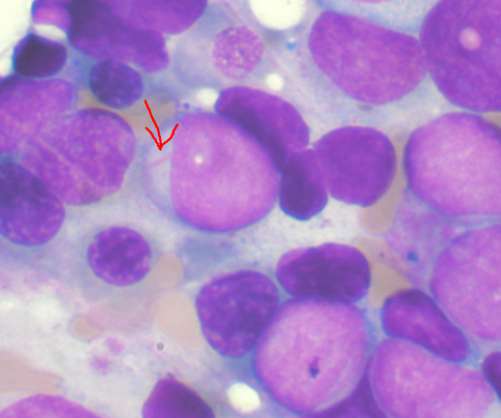
The iPSC-based allogeneic cell therapy platform of Umoja leverages its synthetic receptor enabled differentiation (ShRED) manufacturing process to guide iCIL differentiation and development with robust anti-tumour activity. In the initial stage, the partnership will focus on acute myeloid leukaemia (AML) to boost accessibility to patients.





































Let's personalize your content