A startup launches with plans to open up a gene and cell therapy bottleneck
Bio Pharma Dive
JANUARY 31, 2023
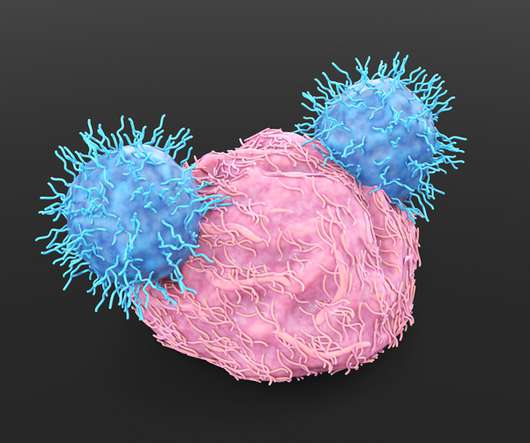
The pipeline of CAR-T therapies and ex vivo gene therapies has swelled in recent years, but manufacturing hasn’t been able to keep up with demand.











































Let's personalize your content