Orphagen’s ACC therapy receives FDA rare pediatric disease status
Pharmaceutical Technology
JANUARY 17, 2023
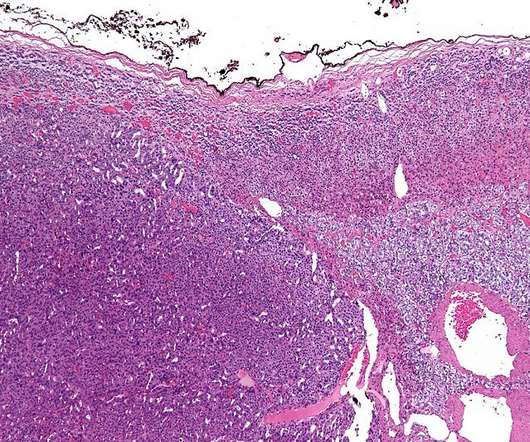
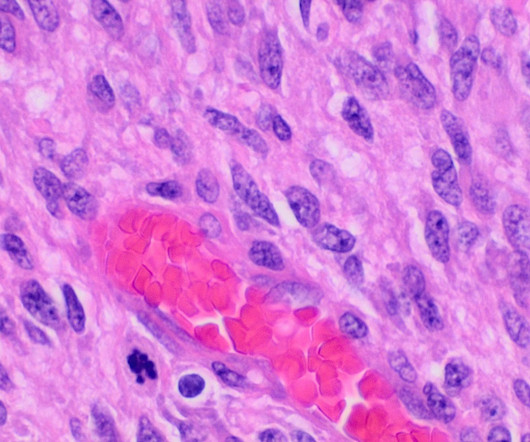
OR-449 is a first-in-class, orally bioavailable, potent and selective small molecule antagonist of the orphan nuclear receptor steroidogenic factor-1 (SF-1, NR5A1). In paediatric ACC, the transcription factor is commonly amplified at the gene level. SF-1 is an important transcription factor for adrenal gland growth and development.

















Let's personalize your content