Merck and Moderna partner to develop and sell cancer vaccine
Pharmaceutical Technology
OCTOBER 14, 2022
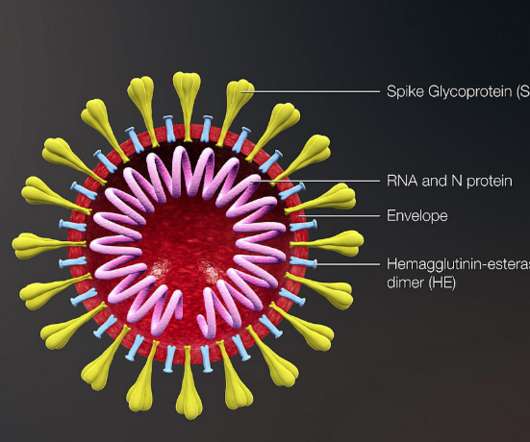
German pharmaceutical firm Merck has extended its partnership with Moderna to jointly develop and sell mRNA-4157/V940, an investigational personalised cancer vaccine (PCV). In 2016, the companies entered a strategic partnership to develop novel messenger RNA (mRNA) based PCVs.






































Let's personalize your content