Adcentrx raises funds to advance ADCs into clinical development
Pharmaceutical Technology
APRIL 26, 2023
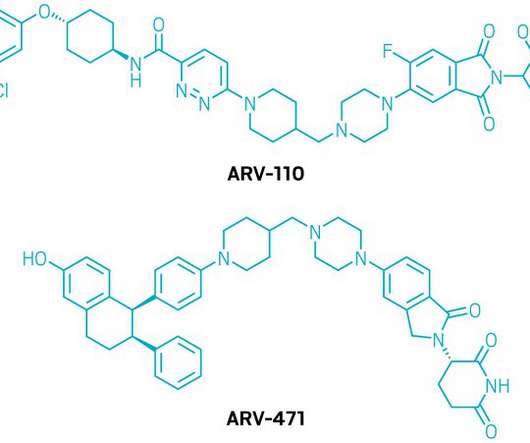
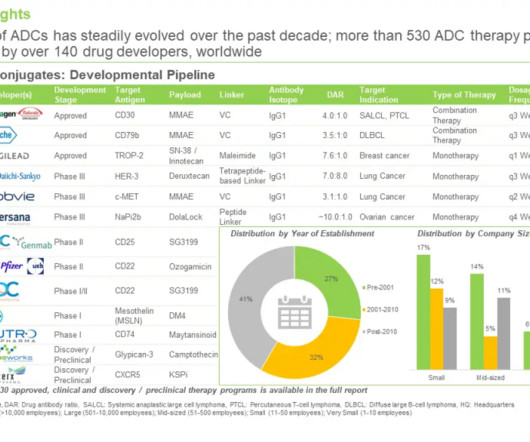
Biotechnology company Adcentrx Therapeutics has raised $38m in Series A+ financing to advance its pipeline of new antibody-drug conjugate (ADC) therapeutics into clinical development. Adcentrx Therapeutics is focused on developing protein conjugate therapeutics to treat cancer and other life-threatening diseases.




































Let's personalize your content