Pfizer seeks approval from US FDA for Covid-19 treatment
Pharmaceutical Technology
JULY 1, 2022
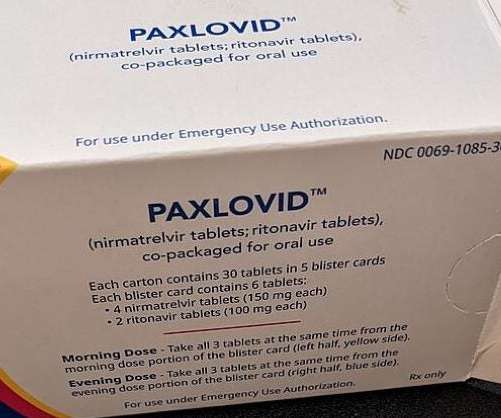
The latest filing is based on non-clinical and clinical data for Paxlovid and also comprised data from the Phase II/III EPIC-HR clinical trial. The safety data currently available for the therapy is in line with over 3,500 Paxlovid-treated subjects across the EPIC clinical development programme.





























Let's personalize your content