Cloud robotics labs are accelerating drug discovery and development
Pharmaceutical Technology
OCTOBER 28, 2022
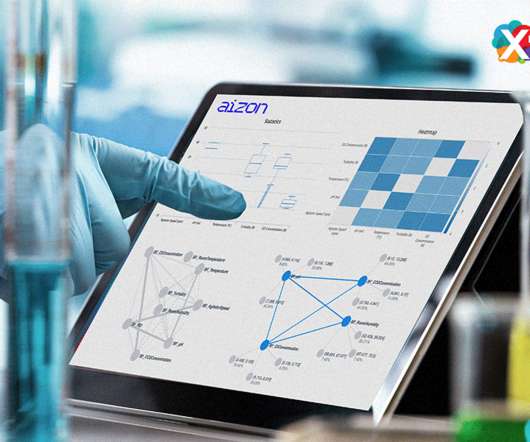
Drug discovery and development is an incredibly expensive and time-consuming process, taking between 12 and 18 years, and costing on average between $2 billion and $3 billion. Robots can improve drug discovery and development. Remote-controlled robotic labs.





























Let's personalize your content