Transforming Clinical Trial Management: The Shift from Point Systems to Platform Solutions
Cloudbyz
MARCH 31, 2024
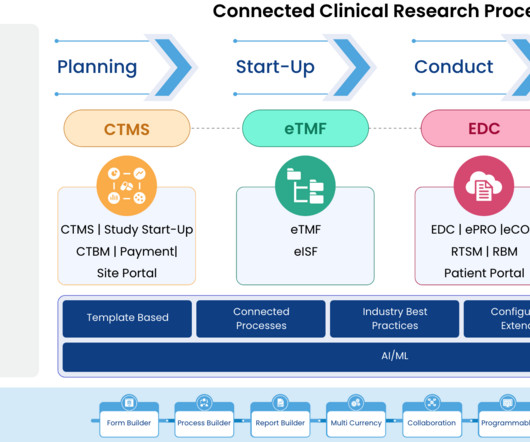
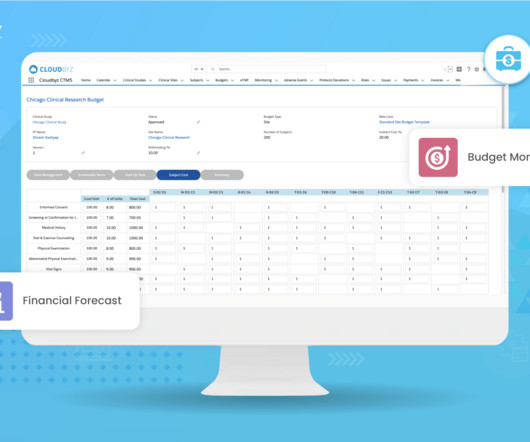
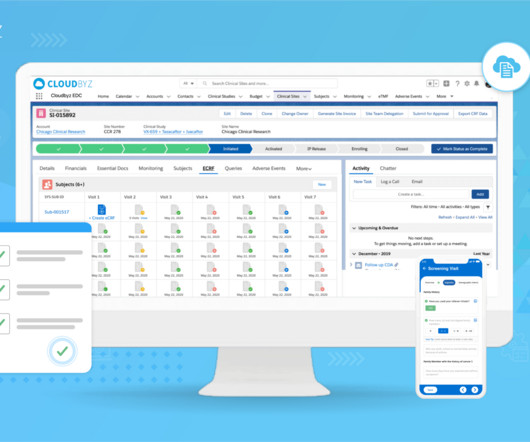
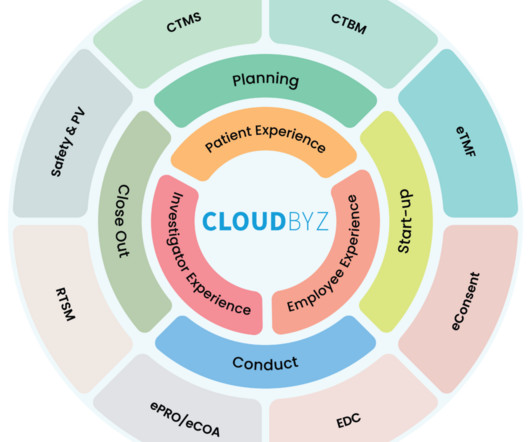
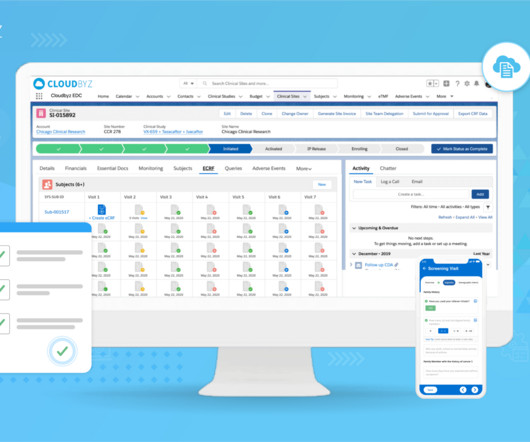
The landscape of clinical trial management is undergoing a significant transformation, driven by the complex demands of modern medical research. As we delve into the challenges posed by point systems, it becomes clear that a platform approach is not just beneficial but essential for the future of clinical trials.












































Let's personalize your content