Wainua (Eplontersen) Now FDA-Approved for Rare Disease ATTRv-PN
XTalks
JANUARY 3, 2024
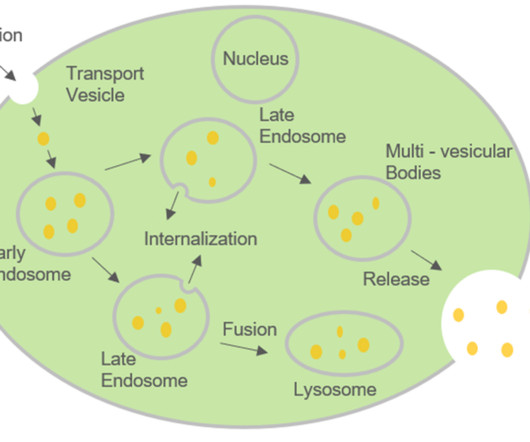
In individuals affected by ATTR, which includes both hereditary and wild-type (non-hereditary) variants, the TTR protein forms fibrils that accumulate in various tissues. These tissues comprise peripheral nerves, the heart, gastrointestinal system, eyes, kidneys, central nervous system, thyroid and bone marrow.



































Let's personalize your content