FDA $7bn plans for 2024: disclose contract manufacturers, restart Cancer Moonshot
Pharmaceutical Technology
MARCH 30, 2023
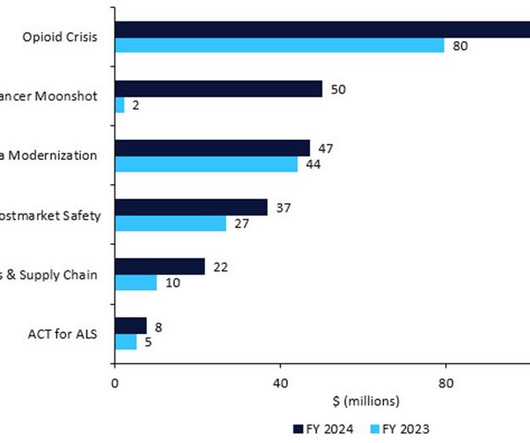
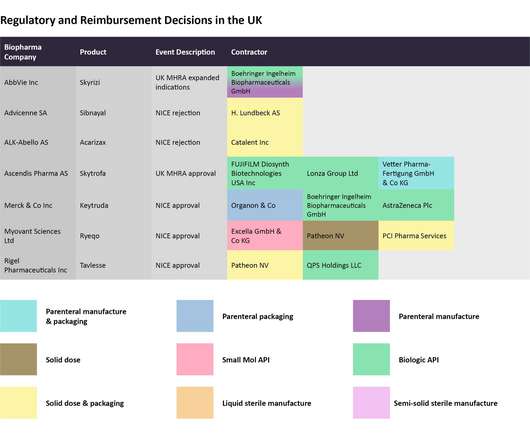
This will be spent on oncology clinical trials with underrepresented subgroups, decentralised trials and real-world evidence, and FDA collaboration with foreign regulators. Restarting Cancer Moonshot: +$48m, for a total of $50m for President Biden’s pet project. Source: FDA © GlobalData






































Let's personalize your content