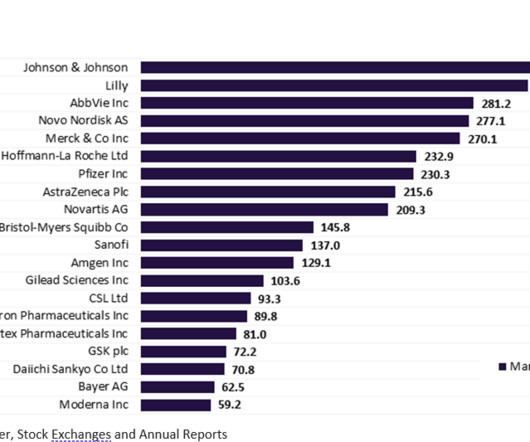
Leading API chemical companies in contract marketing
Pharmaceutical Technology
SEPTEMBER 15, 2022
Various factors have contributed to the need and growth of API chemical suppliers such as rising healthcare expenditure, increasing disposable incomes, growing geriatric population, increasing incidence of chronic diseases, patent expiration of blockbuster drugs, increased consumption of generic drugs, and intervention of the new generation APIs.












































Let's personalize your content