Lilly drug Mounjaro succeeds in second weight loss study
Bio Pharma Dive
APRIL 27, 2023
Trial participants lost up to 15% of their body weight in a finding that should help the closely watched GLP-1 drug gain an FDA approval for obesity.
Bio Pharma Dive
APRIL 27, 2023
Trial participants lost up to 15% of their body weight in a finding that should help the closely watched GLP-1 drug gain an FDA approval for obesity.

Pharmaceutical Technology
APRIL 27, 2023
The European Commission’s (EC) long-anticipated pharma reform plans in the European Union have finally been unveiled , indicating a focus on improving access to medicines across the bloc while cutting down on market exclusivity. Described as the largest reform in over 20 years, the proposed revision touches on multiple topics ranging from unequal access to innovative medicines across the EU to new environmental protections.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
APRIL 27, 2023
The cell and gene therapy developer will also cut back on manufacturing and certain research in California as it redirects resources to three priority programs.
Pharmaceutical Technology
APRIL 27, 2023
Seres Therapeutics and Nestlé Health Science have received approval from the US Food and Drug Administration (FDA) for Vowst (faecal microbiota spores, live-brpk) for preventing the recurrence of C difficile infection (CDI) in adults. Vowst is an orally administered microbiota-based therapeutic, previously known as SER-109, and is indicated for the prevention of recurrence of CDI in people aged 18 years and above following an antibacterial treatment for recurrent CDI (rCDI).
Bio Pharma Dive
APRIL 27, 2023
Partner Regeneron said the data, which showed substantial reduction of a key protein, suggests hope for the “bold dream” of silencing genes in the brain.
Pharmaceutical Technology
APRIL 27, 2023
The UK’s National Institute for Health and Care Excellence (NICE) has recommended two new personalised immunotherapy therapies from Kite Pharma to treat aggressive forms of blood cancer for the Cancer Drugs Fund (CDF). Established in 2011, the CDF is a source of funding to increase patient access to cancer drugs in the UK. The recommended chimeric antigen receptor (CAR) T-cell therapies include Yescarta (Axicabtagene ciloleucel) and Tecartus (Brexucabtagene autoleucel).
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
APRIL 27, 2023
Amicus Therapeutics announced that the Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion for Opfolda (miglustat) intended for treatment of Pompe Disease. The CHMP used evidence from a Phase III trial ( NCT03729362 ) involving 123 patients with late-onset Pompe Disease (LOPD) to adopt a positive opinion for Opfolda.
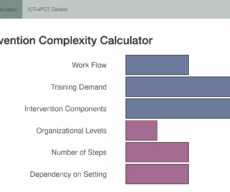
Rethinking Clinical Trials
APRIL 27, 2023
The NIH Pragmatic Trials Collaboratory published a new chapter of its Living Textbook of Pragmatic Clinical Trials this week. The chapter, “ Intervention Delivery and Complexity ,” illustrates that—although an intervention may be simple—the actual delivery of the intervention may be complex due to factors such as new workflows, special training of frontline staff, and the number of components in the intervention.

Pharmaceutical Technology
APRIL 27, 2023
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended the approval of Roche ’s Columvi (glofitamab) to treat relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) in adult patients after receiving two or more lines of systemic therapy. Columvi is an investigational CD20xCD3 T-cell-engaging bispecific antibody designed to target CD20 on the B-cells’ surface and CD3 on the T-cells’ surface.

Bio Pharma Dive
APRIL 27, 2023
Co-founded by physician-researcher Bert Vogelstein in 2021, Haystack raised $56 million in venture financing last November for development of its cancer blood test.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
APRIL 27, 2023
Bristol Myers Squibb will take over a former Novartis plant to boost viral vector production for chimeric antigen receptor (CAR) T-cell therapies. With multi-product, in-house viral vector production capabilities, the new facility in Libertyville, Illinois, US, is expected to strengthen the company’s long-term viral vector supply and advance its long-term goals and global manufacturing network in cell therapy.
Bio Pharma Dive
APRIL 27, 2023
The drugmaker will use a priority review voucher in a bid to secure FDA approval of Mounjaro in obesity by late this year or early next.

Pharmaceutical Technology
APRIL 27, 2023
Novartis has received a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) on Cosentyx (secukinumab) for use in adults with active moderate to severe hidradenitis suppurativa (HS), a painful and persistent inflammatory skin disease. CHMP has also recommended a marketing authorisation for Cosentyx, a fully human biologic that directly inhibits interleukin-17A cytokine.

Medical Xpress
APRIL 27, 2023
Research led by Maynooth University and published today in the journal Science Signaling has found that the protein MYC is essential for MAIT cell growth, proliferation, metabolism and function. The latest findings are of critical importance to the study of MAIT cells and the development of metabolic disease such as obesity.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
APRIL 27, 2023
Evommune has received $50m in Series B financing to accelerate the development of therapies for the treatment of chronic inflammatory diseases. Co-led by new investor Arix Bioscience and current investors EQT Life Sciences and SymBiosis, the financing round also included new and existing investors such as Amplitude Ventures. Pivotal bioVenture Partners and Andera Partners also participated in the company’s Series A funding round.

STAT News
APRIL 27, 2023
In public, hospitals rave about artificial intelligence. They trumpet the technology in press releases, plaster its use on billboards, and sprinkle AI into speeches touting its ability to detect diseases earlier and make health care faster, better, and cheaper. But on the front lines, the hype is smashing into a starkly different reality.
Pharmaceutical Technology
APRIL 27, 2023
Orbital Therapeutics has raised $270m in a Series A round led by ARCH Venture Partners to advance a portfolio of programmable RNA therapeutics. The initial investors were a16z Bio + Health and Newpath Partners. New investors included the Redmile Group, the Abu Dhabi Growth Fund (ADG), Exor, Invus, Moore Strategic Ventures, the iGlobe Platinum Fund Group and Casdin Capital.

Rethinking Clinical Trials
APRIL 27, 2023
Speakers Amit Garg, MD, MA (Education) FRCPC, FACP, PhD Associate Dean, Clinical Research, Schulich School of Medicine and Dentistry Lead, Institute for Clinical Evaluative Sciences Kidney, Dialysis and Transplantation Provincial Program Director, Institute for Clinical Evaluative Sciences (ICES) Western Facility Nephrologist, London Health Sciences Centre Professor, Medicine, Epidemiology & Biostatistics,

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Medical Xpress
APRIL 27, 2023
A plant-based compound purified from the traditional Chinese herb, Astragalus, has the potential to improve the outcome of heart attack patients, new research has revealed.
ProRelix Research
APRIL 27, 2023
Pharmacovigilance is defined by the World Health Organization (WHO) as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse events or any other drug-related problems.” […] The post Pharmacovigilance Services for Signal Management: Process as per US FDA appeared first on ProRelix Research.

Medical Xpress
APRIL 27, 2023
U.S. health officials on Wednesday approved the first pill made from healthy bacteria found in human waste to fight dangerous gut infections—an easier way of performing so-called fecal transplants.

STAT News
APRIL 27, 2023
Editor’s Note: A livestream of the event will be embedded below at 1 p.m. ET. Each year, STAT chooses a new class of Wunderkinds, showcasing stars of science and medicine who are in the midst of launching their careers.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Medical Xpress
APRIL 27, 2023
U.S. cigarette smoking dropped to another all-time low last year, with 1 in 9 adults saying they were current smokers, according to government survey data released Thursday. Meanwhile, electronic cigarette use rose, to about 1 in 17 adults.

XTalks
APRIL 27, 2023
care.ai , a smart healthcare technology company that offers autonomous monitoring solutions for hospitals and clinics, has announced a partnership with Google Cloud. The collaboration will allow care.ai to leverage Google Cloud’s infrastructure and machine learning capabilities to enhance its healthcare solutions, including its AI-driven Smart Care Facility Platform.

Medical Xpress
APRIL 27, 2023
An international research team led by investigators at Virginia Commonwealth University has identified for the first time markers that may indicate early in life if a person has susceptibility to schizophrenia.

STAT News
APRIL 27, 2023
You’re reading the web edition of STAT Health Tech, our guide to how tech is transforming the life sciences. Sign up to get this newsletter delivered in your inbox every Tuesday and Thursday.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Drug Discovery World
APRIL 27, 2023
This is the latest episode of the free DDW narrated podcast, “The impact of technology on drug discovery”. It covers two articles written for Volume 23, Issue 2 – Spring 2022 of DDW. They are called “ Talking Tech ” and “ Rejuvenation biotech: Can this company make age a thing of the past? ” With Covid-19 taking so much of the drug discovery and development’s focus over the last two years, it has been easy to overlook other areas within the sector that deserve our attention.
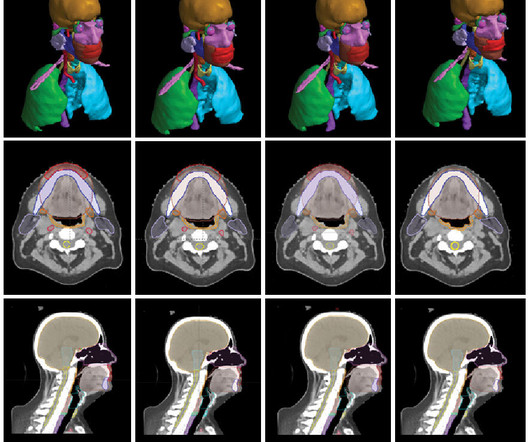
Medical Xpress
APRIL 27, 2023
A study conducted by Mayo Clinic suggests artificial intelligence could potentially improve time efficiency and standardization for radiation therapy planning in patients with head and neck cancers. The validation study, published in Frontiers in Oncology, evaluated the efficiency of an algorithm trained by Mayo Clinic and developed in collaboration with Google Health.

JAMA Internal Medicine
APRIL 27, 2023
This cross-sectional study evaluates the ability of an artificial intelligent chatbot to provide quality and empathetic responses to patient questions.
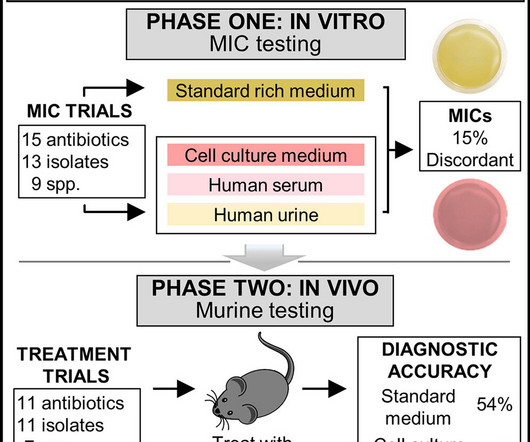
Medical Xpress
APRIL 27, 2023
A new test revealed that FDA-approved antibiotics—available at your neighborhood pharmacy—can effectively treat superbugs. They are not prescribed, however, because the gold-standard test predicts they will not work. The new test may improve the way antibiotics are developed, tested and prescribed—and it is openly available to all.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content