Final obstacles in front of Pfizer, Seagen merger fall
pharmaphorum
DECEMBER 13, 2023
Final obstacles in front of Pfizer, Seagen merger fall Phil.

pharmaphorum
DECEMBER 13, 2023
Final obstacles in front of Pfizer, Seagen merger fall Phil.

Bio Pharma Dive
DECEMBER 13, 2023
The company is now expecting to save $4 billion by the end of 2024, with a majority of the spending cuts coming from drug research and development.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
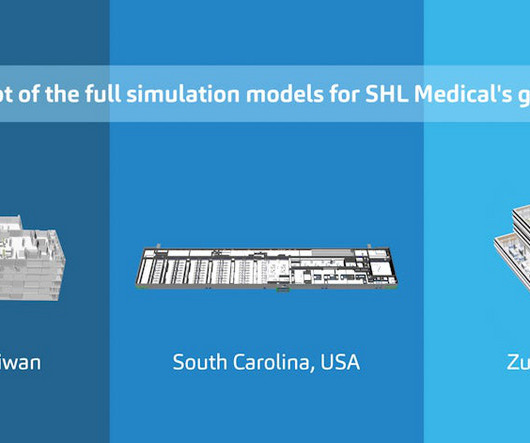
Pharmaceutical Technology
DECEMBER 13, 2023
Advanced simulation methods and tools are not only paving the way for more efficient manufacturing operations. At SHL Medical, they’re also speeding up the development of new production facilities around the globe.

Bio Pharma Dive
DECEMBER 13, 2023
The closely watched medicine showed promise treating a form of chronic nerve pain, with the results suggesting comparable effects to an available therapy.

Rethinking Clinical Trials
DECEMBER 13, 2023
In this Friday's PCT Grand Rounds, Roxana Mehran of the Icahn School of Medicine at Mount Sinai will present "Diversifying Clinical Trials: A Path Forward." The Grand Rounds session will be held on Friday, December 15, 2023, at 1:00 pm eastern. Mehran is a professor of medicine and the director of interventional cardiovascular research and clinical trials at the Zena and Michael A.

Bio Pharma Dive
DECEMBER 13, 2023
Uptake of Casgevy and Lyfgenia may be slow despite their dramatic benefit, physicians said, citing complexities in treatment, manufacturing and reimbursement.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
DECEMBER 13, 2023
The court's decision on mifepristone, expected by June, could have far reaching implications for the FDA as well as the biotechnology industry.

Pharmaceutical Technology
DECEMBER 13, 2023
The sale, which will see Aditxt take on Evofem’s debt, follows a strategic review by the women’s health company earlier this year.
Fierce Pharma
DECEMBER 13, 2023
Using Tuesday to announce good news—that its $43 billion acquisition of Seagen would be | Using Tuesday to announce good news—that its $43 billion acquisition of Seagen would be finalized on Thursday—Pfizer sandwiched its bad news, which came on Wednesday with its 2024 guidance. The company expects revenue to reach between $58.5 billion and $61.5 billion next year, coming up short of the analyst consensus of $63.2 billion, largely because of the plummeting demand for its COVID-19 products.
Pharmaceutical Technology
DECEMBER 13, 2023
Daiichi Sankyo plans to leverage the French startup’s technology to develop its RNA-targeted therapeutics pipeline.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharma Times
DECEMBER 13, 2023
In England, ruptured brain aneurysms occur in around one in 15,000 people every year - News - PharmaTimes

Pharmaceutical Technology
DECEMBER 13, 2023
AstraZeneca has signed a definitive agreement to initiate a tender offer for the acquisition of Icosavax for an equity price of $1.1bn.

XTalks
DECEMBER 13, 2023
As we step into 2024, the food industry is poised to be at the cusp of transformative changes, shaped by evolving consumer preferences, technological advancements and a growing emphasis on sustainability. Here, we delve into the 2024 food industry trends from leading experts in the field, each offering a unique perspective on the trends and challenges that will define the landscape of food production, distribution and consumption in the coming year.

Pharmaceutical Technology
DECEMBER 13, 2023
Freya Biosciences has secured $38m in a Series A funding round to progress the development of women’s reproductive immunotherapies.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharma Times
DECEMBER 13, 2023
The software has reduced the time to provide pathology data from days to hours - News - PharmaTimes

Pharmaceutical Technology
DECEMBER 13, 2023
The Japanese MHLW has accepted for review GSK’s regulatory application for the RSV vaccine, Arexvy, in high-risk adults aged 50-59.

XTalks
DECEMBER 13, 2023
The US Food and Drug Administration (FDA) has approved the first gene therapies for the treatment of sickle cell disease, approving two on the same day. The landmark approvals were awarded to bluebird bio’s Lyfgenia (lovo-cel) and Vertex Pharmaceuticals and CRISPR Therapeutics’ jointly developed Casgevy (exa-cel). Both gene therapies are approved for individuals 12 years of age and older with sickle cell disease.

Pharmaceutical Technology
DECEMBER 13, 2023
Top five technology investment trends to watch in 2024 including AI, cloud computing, cybersecurity, IoT and robotics

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Commerce
DECEMBER 13, 2023
The seminar examines where members of the pharma supply chain stand in terms of preparation, being that the act has officially been enacted.
Pharmaceutical Technology
DECEMBER 13, 2023
Odronextamab has demonstrated clinically meaningful efficacy and safety in patients with R/R FL and DLBCL.

Fierce Pharma
DECEMBER 13, 2023
After last year’s explosive ruling to overturn the historic abortion ruling reached in Roe v. Wade, abortion access is back up for debate in the Supreme Court. | The Supreme Court's final decision could have consequences beyond abortion access. That's because the original verdict questioned the FDA's decision-making authority, prompting amicus brief submissions from high-profile industry players.

Pharmaceutical Technology
DECEMBER 13, 2023
Ironwood Pharmaceuticals completed an acquisition of the gastrointestinal disease company VectivBio.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

BioPharma Reporter
DECEMBER 13, 2023
Lisa Sellers is the CEO of Vector Laboratories, and she is passionate about staying ahead of the pharma trends.

pharmaphorum
DECEMBER 13, 2023
Eisai and Biogen’s Alzheimer’s disease therapy Leqembi is due to be launched in Japan, its second market, on 20th December, and will be priced quite a lot lower there than in the US. The anti-amyloid antibody has been included in the Japan National Health Insurance (NHI) Drug Price List at just under JPY 3 million (around $20,500) per patient per year, whereas in the US its list price before discounts or rebates was set at $26,500.

BioSpace
DECEMBER 13, 2023
What needs to happen for funding in biopharma to bounce back? BioSpace’s Lori Ellis discuss the macroeconomic environment and biopharma funding outlook with venture capital guests Ansbert Gadicke, Martin Gershon and Mike Goguen.

pharmaphorum
DECEMBER 13, 2023
The Supreme Court of the US has said it will consider a lower court ruling that would restrict access to the abortion pill mifepristone and is considered to be tied to the standing and authority of the FDA.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

BioSpace
DECEMBER 13, 2023
Being laid off, experiencing a trial failure or failing to secure funding can feel crushing. Here’s how to maintain perspective and move on.

Drug Channels
DECEMBER 13, 2023
This week, I’m rerunning some popular posts while I prepare for Fiday's Drug Channels Outlook 2024 live video webinar. Click here to see the original post from September 2023. Time for our annual update on the channels for provider-administered drugs. For 2023, specialty pharmacies—via white, brown, and clear bagging—retained a meaningful share of the distribution channels for provider-administered oncology drugs.

Fierce Pharma
DECEMBER 13, 2023
After wrapping up a $200 million renovation at a former Boehringer Ingelheim plant near Cleveland, Ohio, several years back, Danish specialty drugmaker Xellia Pharmaceuticals is paring back its wor | After wrapping up a $200 million renovation at a former Boehringer Ingelheim plant near Cleveland, Ohio, several years back, Danish specialty drugmaker Xellia Pharmaceuticals is paring back its workforce in The Forest City.

BioSpace
DECEMBER 13, 2023
The combination of Keytruda and an mRNA vaccine reduced the risk of death or relapse in patients with the most deadly form of skin cancer after three years in a Phase IIb study.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content