FDA panel backs Bluebird gene therapy despite safety risks
Bio Pharma Dive
JUNE 9, 2022
Advisers to the agency voted 15-0 in support of the rare disease treatment eli-cel, which was linked to a bone marrow cancer in three study participants.

Bio Pharma Dive
JUNE 9, 2022
Advisers to the agency voted 15-0 in support of the rare disease treatment eli-cel, which was linked to a bone marrow cancer in three study participants.

pharmaphorum
JUNE 9, 2022
Sanofi has launched a new initiative in its drive to integrate digital technologies throughout its business, setting up an accelerator based in Paris that will serve as the lynchpin of the effort. The accelerator has been set up to “develop products and solutions that will support Sanofi’s mission to transform the practice of medicine with the use of digital, data and artificial intelligence (AI)”, said the drugmaker.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JUNE 9, 2022
Once among biotech’s most valuable companies, Bluebird is running out of cash. Its fate could rest on the FDA’s review of two rare disease treatments, which are being discussed at a two-day meeting beginning Thursday.

BioSpace
JUNE 9, 2022
The House of Representatives voted on a bill that would give the U.S. Food and Drug Administration more power to ensure biopharma companies run large follow-up trials to confirm accelerated approvals.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Bio Pharma Dive
JUNE 9, 2022
An experimental medicine the company is developing with Regeneron has shown early promise treating IgA nephropathy, a disease that’s become a competitive target among drugmakers.

BioSpace
JUNE 9, 2022
The European Hematology Association’s 2022 Hybrid Congress is in full swing in Vienna, Austria and will feature the newest and most promising developments in blood cancer research.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
BioSpace
JUNE 9, 2022
For the 37.3 million Americans who have diabetes, insulin has become increasingly expensive. Here are four organizations dedicated to making insulin more accessible.

BioPharma Reporter
JUNE 9, 2022
CureVac has acquired Frame Cancer Therapeutics: with the German mRNA specialist planning to draw on Frameâs expertise in antigen identification and validation as it develops mRNA cancer vaccines.

BioSpace
JUNE 9, 2022
Following a marathon session of the FDA's Cell, Tissue and Gene Therapies Advisory Committee, bluebird bio passed its first critical hurdle in approval for the lentiviral vector gene therapy, eli-cel.

XTalks
JUNE 9, 2022
Israeli food tech startup Yo! Egg, which has developed plant-based poached and sunny-side-up eggs, announced that it plans to launch in US restaurants later this year. In this episode of the Xtalks Food Podcast, Sydney introduces the team to Yo! Egg’s plant-based “whole egg” alternative, which is made up of two parts — the “white” and the “yolk.” While the taste and texture of Yo!

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

BioSpace
JUNE 9, 2022
Location is everything when it comes to compensation in the life sciences. We've compiled data from BioSpace's 2022 Life Sciences salary report to help you choose the best place to live and work.
Scienmag
JUNE 9, 2022
HAMILTON, June 10, 2022–McMaster University scientists who compared respiratory vaccine-delivery systems have confirmed that inhaled aerosol vaccines provide far better protection and stronger immunity than nasal sprays. Credit: McMaster University HAMILTON, June 10, 2022–McMaster University scientists who compared respiratory vaccine-delivery systems have confirmed that inhaled aerosol vaccines provide far better protection and stronger immunity than […].
BioSpace
JUNE 9, 2022
Bavarian Nordic announced the U.S. Biomedical Advanced Research and Development Authority has ordered an additional 500,000 doses of its monkeypox vaccine, Jynneos.

Scienmag
JUNE 9, 2022
Researchers at the University of Queensland have found a species of worm with an appetite for polystyrene could be the key to plastic recycling on a mass scale. Scientists discovered the common Zophobas morio ‘superworm’ can eat through polystyrene, thanks to a bacterial enzyme in their gut. Credit: The University of Queensland Researchers at the University of […].

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
BioSpace
JUNE 9, 2022
Researchers from all around the world have been working on mapping human genes to their functions. Now, for the first time, that mapping has been completed.

pharmaphorum
JUNE 9, 2022
Antimicrobial resistance (AMR) is an issue of global concern, killing roughly 1.2 million 1 people per year and projected to rise to 10 million people by 2050 2. It occurs when bacterial pathogens adapt over time to develop resistance to traditional antibiotics, resulting in “superbugs” that are near impossible to treat. In order to reduce the global healthcare impact of the evolving AMR situation, companies such as Micreos Pharmaceuticals are taking a novel and highly innovative approach to ant

Scienmag
JUNE 9, 2022
After a six-year journey, a plucky spacecraft called Hayabusa2 zinged back into Earth’s atmosphere in late 2020 and landed deep in the Australian outback. When researchers from the Japanese space agency JAXA opened it, they found its precious payload sealed and intact: a handful of dirt that Hayabusa2 managed to scoop off the surface of […].
Pharma Times
JUNE 9, 2022
Vital dengue vaccine offers over four years’ protection following successful clinical trial

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Scienmag
JUNE 9, 2022
Investments in the environment are paying off for a California county where projects designed to restore the natural environment are also buffering the impacts of sea-level rise, according to a new study by Stanford researchers. The research, published June 9 in Urban Sustainability, shows that nature-based solutions, such as conserving marshlands and restoring beaches, can be […].

BioSpace
JUNE 9, 2022
GSK reported positive headline data from a pre-specified efficacy interim analysis of the Phase III trial of its RSV vaccine.

Scienmag
JUNE 9, 2022
A new molecule synthesized by a University of Texas at Dallas researcher kills a broad spectrum of hard-to-treat cancers, including triple-negative breast cancer, by exploiting a weakness in cells not previously targeted by other drugs. Credit: The University of Texas at Dallas A new molecule synthesized by a University of Texas at Dallas researcher kills […].

Drug Channels
JUNE 9, 2022
Today’s guest post comes from Edward Hensley, Co-founder and Chief Commercial Officer at AssistRx. Edward offers five ways life sciences companies can use technology to transform their patient support services and thus differentiate their products. To learn more, sign up for AssistRx’s free webinar Specialty Pharma’s 2022 Patient Support Trends: Progress Check.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Scienmag
JUNE 9, 2022
Wreck of historic royal ship discovered off the English coast Credit: University of East Anglia Wreck of historic royal ship discovered off the English coast The wreck of one of the most famous ships of the 17th century – which sank 340 years ago while carrying the future King of England James Stuart – has been […].

BioSpace
JUNE 9, 2022
Researchers have linked areas of the brain with people who have takotsubo syndrome, also known as broken heart syndrome.
Scienmag
JUNE 9, 2022
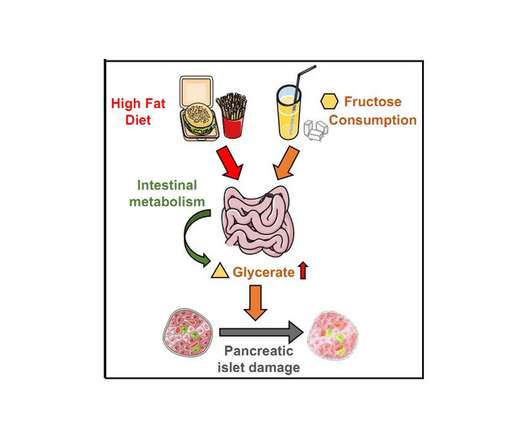
(LOS ANGELES) – June 9, 2022 – Those who are habitually inclined to consume burgers, fries and soda may think twice about their dietary choices following scientists’ latest findings about high-fat, high-fructose diets. Credit: Cell Metabolism (LOS ANGELES) – June 9, 2022 – Those who are habitually inclined to consume burgers, fries and soda may […].
pharmaphorum
JUNE 9, 2022
The UK’s fertile environment for biotechs specialising in artificial intelligence has generated a new player – Charm Therapeutics – which came out of stealth mode this morning with a $50 million first-round financing. The London-based start-up has been founded by David Baker, head of the Institute of Protein Design at the University of Washington in Seattle, and Laksh Aithani, whose past roles include a couple of years working on the machine learning platform of fellow UK-based AI speciali

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Scienmag
JUNE 9, 2022
NEW YORK, JUNE 9, 2022 – In biology, getting rid of stuff can be just as important as making it. A buildup of cells, proteins, or other molecules that are no longer needed can cause problems, so living things have evolved several ways to clean house. Credit: Memorial Sloan Kettering Cancer Center NEW YORK, JUNE […].

Clairnes
JUNE 9, 2022
The post Decentralized Clinical Trials: the next step towards digitalization of clinical research appeared first on Clinical Trial Recruitment & Management Services.

Scienmag
JUNE 9, 2022
PHILADELPHIA – The behind-the-scenes story detailing the pursuit of a transformative cancer cure will unfold onscreen at the Tribeca Film Festival in New York City this weekend. “Of Medicine and Miracles,” which will premiere during the renowned international festival, is an emotional journey, revealing decades of research – and one young patient’s family’s last hopes […].

Pharma Times
JUNE 9, 2022
Agreement between the two companies includes process development of AAV-based gene therapies

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content