Iowa State University Approaches COVID-19 Using Nanotechnology
BioSpace
DECEMBER 27, 2020
The vaccine will be able to be administered without needles and in one dose. It also won’t require refrigeration.
BioSpace
DECEMBER 27, 2020
The vaccine will be able to be administered without needles and in one dose. It also won’t require refrigeration.

JAMA Internal Medicine
DECEMBER 27, 2020
This cohort study uses data from the electronic health record of patients admitted to Cleveland Clinic Hospitals to assess the outcomes of adults with noncardiac inpatient admissions who were treated for hypertension.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioSpace
DECEMBER 27, 2020
The United Kingdom is expected to give the green light to the vaccine developed by AstraZeneca and Oxford University this week.

JAMA Internal Medicine
DECEMBER 27, 2020
This comparative effectiveness study assesses whether the health outcomes of White US citizens living in the 1% and 5% richest counties are better than the health outcomes of average residents in other developed countries.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

BioSpace
DECEMBER 27, 2020
Shares of Aprea Therapeutics plunged more than 77% in morning trading after the company announced its late-stage cancer combination treatment failed to meet its primary endpoint of complete remission rate.

Delveinsight
DECEMBER 27, 2020
Characterized by debilitating episodes of depression and mood elevation (mania or hypomania), Bipolar depression is an under-recognized and unappreciated phase of bipolar disorder. Since most patients exhibit depression, Bipolar depression gets misdiagnosed with other mental disorders easily. Thus, it is associated with a heavier burden of illness, morbidity, and cost.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

The Pharma Data
DECEMBER 27, 2020
STOCKHOLM , Dec. 28, 2020 /PRNewswire/ — C-RAD announces today that C-RAD has been selected as the partner to implement surface tracking technology for three proton cancer treatment centers in the US. C-RAD offers a specific version of its Catalyst System for use in proton and particle therapy – Catalyst PT. It is based on the patented Catalyst technology and software as well as the hardware are tailored for the application in this environment. The Catal

BioSpace
DECEMBER 27, 2020
Myovant Sciences’ stock rocketed 22% on news it was partnering with Pfizer to develop and commercialize relugolix for prostate cancer and women’s health.

The Pharma Data
DECEMBER 27, 2020
–Received Gross Proceeds of $110 Million from Previously Announced PIPE Financing– –Executed Additional PIPE Financing of $15 Million– EMERYVILLE, Calif., Dec. 28, 2020 (GLOBE NEWSWIRE) — Gritstone Oncology , Inc. (Nasdaq: GRTS), a clinical-stage biotechnology company developing the next generation of cancer immunotherapies to fight multiple cancer types, today announced the closing of the previously announced $110 million private investment in public equity (

BioSpace
DECEMBER 27, 2020
Even with the holidays among us, there were a number of clinical trial announcements. Here’s a look.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

The Pharma Data
DECEMBER 27, 2020
LUND, Sweden , Dec. 28, 2020 /PRNewswire/ — Alligator Bioscience (Nasdaq Stockholm: ATORX) today announced that it has submitted a CTA (Clinical Trial Authorization) application to the relevant regulatory authorities to start a Phase II efficacy study of its wholly-owned CD40 targeting antibody mitazalimab. The upcoming Phase II study OPTIMIZE-1 is an open-label, multi-center study assessing the clinical efficacy of mitazalimab in combination with chemotherapy (mFolfirinox) in patients wit
Drug Patent Watch
DECEMBER 27, 2020
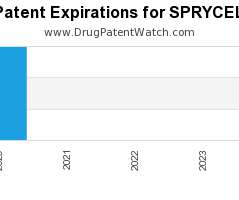
Annual Drug Patent Expirations for SPRYCEL Sprycel is a drug marketed by Bristol Myers Squibb and is included in one NDA. It is available from one supplier. There are five…. The post New patent expiration for Bristol Myers drug SPRYCEL appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
DECEMBER 27, 2020
Voucher expected to be applied to the forthcoming NDA for Tyvaso DPI. SILVER SPRING, Md. and RESEARCH TRIANGLE PARK, N.C. , Dec. 28, 2020 /PRNewswire/ — United Therapeutics Corporation (Nasdaq: UTHR) announced today an agreement to acquire a Rare Pediatric Disease Priority Review Voucher (PRV), which it plans to use with a forthcoming New Drug Application (NDA) with the U.S.

BioTech 365
DECEMBER 27, 2020
BeiGene Announces Inclusion of Three Innovative Oncology Products in China National Reimbursement Drug List (NRDL) BeiGene Announces Inclusion of Three Innovative Oncology Products in China National Reimbursement Drug List (NRDL) Internally-developed anti-PD-1 antibody tislelizumab and BTK inhibitor BRUKINSA® (zanubrutinib) are … Continue reading →

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

The Pharma Data
DECEMBER 27, 2020
Dec. 28, 2020 02:43 UTC. BEIJING–( BUSINESS WIRE )– InnoCare Pharma (HKEX: 09969), a leading biopharmaceutical company, announced today that its BTK inhibitor orelabrutinib received approval from the China National Medical Products Administration (NMPA) in two indications: the treatment of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) /small lymphocytic lymphoma (SLL), and the treatment of patients with relapsed/refractory mantle cell lymphoma (MCL).

BioTech 365
DECEMBER 27, 2020
Orphazyme provides regulatory update on arimoclomol for NPC Orphazyme provides regulatory update on arimoclomol for NPC Orphazyme A/SCompany announcement … Continue reading →

The Pharma Data
DECEMBER 27, 2020
In 2020, messenger RNA (mRNA), the technology behind Pfizer’s and Moderna’s COVID-19 vaccine, practically became a household word. Now the gate is open for more mRNA vaccines to flood the space. Source link.

BioTech 365
DECEMBER 27, 2020
InnoCare Announces the Approval of Orelabrutinib in China for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma and Relapsed/Refractory Mantle Cell Lymphoma InnoCare Announces the Approval of Orelabrutinib in China for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia or … Continue reading →

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

The Pharma Data
DECEMBER 27, 2020
Swedish drugmaker Vicore Pharma has reported positive results from a phase 2 study evaluating its oral therapy for treating respiratory infection in hospitalized COVID-19 patients. Source link.

BioTech 365
DECEMBER 27, 2020
Cacao Bliss Reviews 2021 – Danette May and Earth Echo Cacao Bliss Review Report by FitLivings Cacao Bliss Reviews 2021 – Danette May and Earth Echo Cacao Bliss Review Report by FitLivings Earth Echo Cacao Bliss reviews update.

The Pharma Data
DECEMBER 27, 2020
SUNDAY, Dec. 27, 2020 — Outdoor activities can help you keep fit this winter while staying safe from COVID-19, but you need to take precautions to reduce your risk of injury, an expert says. Skiing and snowboarding are good examples. Falls are common in these sports, but proper technique and safety gear can reduce the risk of injury. Each year, nearly 120,000 ski- and snowboard-related injuries are treated in U.S. emergency rooms, doctors’ offices and clinics, according to the U.S.

BioTech 365
DECEMBER 27, 2020
ProMind Complex Reviews 2021 – Ingredients Really Work? Review by FitLivings ProMind Complex Reviews 2021 – Ingredients Really Work? Review by FitLivings ProMind Complex supplement reviews.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

The Pharma Data
DECEMBER 27, 2020
. EMERYVILLE, Calif. , Dec. 28, 2020 /PRNewswire/ — Amyris, Inc. (Nasdaq: AMRS), a leading synthetic biotechnology company active in the Clean Health and Beauty markets through its consumer brands, and a top supplier of sustainable and natural ingredients, today announced that it has received Bonsucro Chain of Custody Certification. Bonsucro is a global organization that promotes sustainable sugarcane production, processing and trade around the world.

The Pharma Data
DECEMBER 27, 2020
Dec. 28, 2020 13:00 UTC. CARLSBAD, Calif.–( BUSINESS WIRE )– Lineage Cell Therapeutics, Inc. (NYSE American and TASE: LCTX), a clinical-stage biotechnology company developing allogeneic cell therapies for unmet medical needs, today provided a year-end review and an outline of its plans for 2021. To Our Shareholders, As we reach the end of an eventful 2020, we are inspired by the many healthcare providers and biopharmaceutical companies that worked to combat the COVID-19 pandemic.
Drug Patent Watch
DECEMBER 27, 2020
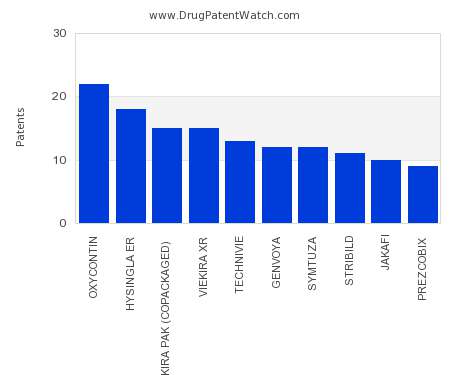
This chart shows the drugs with the most patents in Croatia. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Croatia? appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

The Pharma Data
DECEMBER 27, 2020
Dec. 28, 2020 07:00 UTC. PARIS–( BUSINESS WIRE )– Regulatory News: Lysogene (FR0013233475 – LYS) (Paris:LYS), a phase 3 gene therapy platform company targeting central nervous system (CNS) diseases, today reports positive biomarker data from the ongoing AAVance clinical trial with LYS-SAF302 for the treatment of MPS IIIA (NCT03612869). Changes in heparan sulfate (HS) concentration in cerebrospinal fluid (CSF) are being monitored in patients treated with LYS-SAF302 to provide evidence

The Pharma Data
DECEMBER 27, 2020
Even with the holidays among us, there were a number of clinical trial announcements. Here’s a look. COVID-19-Related. Vir Biotechnology and GlaxoSmithKline dosed the first patient in a new sub-trial of a Phase III study of monoclonal antibody VIR-7831 for hospitalized adults with COVID-19. RedHill Biopharma ’ s Phase II/III trial of opaganib in severe COVID-19 pneumonia received a second unanimous recommendation to continue by a second IDSMB safety review.

The Pharma Data
DECEMBER 27, 2020
SHANGHAI , Dec. 27, 2020 /PRNewswire/ — Gannex , a wholly owned company of Ascletis Pharma Inc. (HKEX: 1672) and fully dedicated to the R&D and commercialization of new drugs in the field of NASH, announces today dosing of first subject with its NASH drug candidate ASC42, a Farnesoid X Receptor (FXR) agonist, in a U.S. Phase I Trial. This U.S. phase I trial is a randomized, double-blind, placebo-controlled, single and multiple dose escalation study to evaluate safety, tolerability, pha

The Pharma Data
DECEMBER 27, 2020
SOUTH SAN FRANCISCO, Calif. and TAIPEI, Taiwan, Dec. 28, 2020 (GLOBE NEWSWIRE) — TLC (Nasdaq: TLC, TWO: 4152), a clinical-stage specialty pharmaceutical company developing novel nanomedicines to target areas of unmet medical need, announced today that patient enrollment of EXCELLENCE, the Phase III pivotal clinical trial for TLC599 in patients with osteoarthritis (OA) knee pain, has been completed.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content