The promising role of GLP-1RAs in NASH
Pharmaceutical Technology
JUNE 28, 2023
Research has shown that GLP-1RAs may have the potential to treat chronic metabolic diseases such as non-alcoholic steatohepatitis (NASH).

Pharmaceutical Technology
JUNE 28, 2023
Research has shown that GLP-1RAs may have the potential to treat chronic metabolic diseases such as non-alcoholic steatohepatitis (NASH).
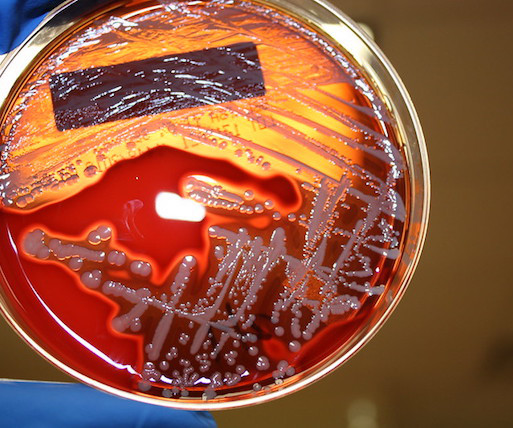
AuroBlog - Aurous Healthcare Clinical Trials blog
JUNE 28, 2023
Golden staph are ubiquitous bacteria, living harmlessly on the skin or inside the nose of nearly one in three people worldwide. Yet they are duplicitous, too. In some situations, golden staph (Staphylococcus aureus) can become monsters, causing dangerous infections in the skin, blood, bones, or elsewhere.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
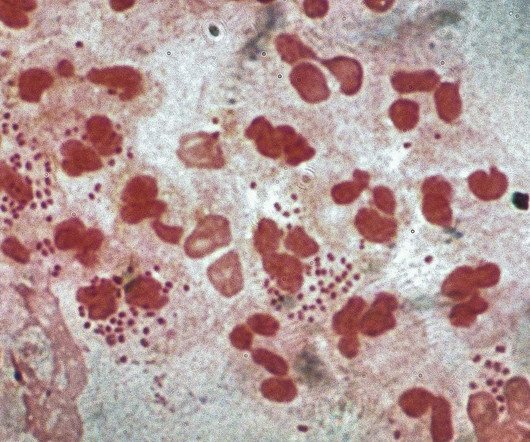
Pharmaceutical Technology
JUNE 28, 2023
GSK has received a Fast Track designation for its Neisseria gonorrhoeae investigational vaccine from the US Food and Drug Administration.
Bio Pharma Dive
JUNE 28, 2023
A manufacturing issue led the agency to turn back an application for a high-dose form of Eylea, surprising analysts and delaying a launch the company is relying on to answer a competitive threat from Roche.
Pharmaceutical Technology
JUNE 28, 2023
Cellusion shares Phase I/II clinical development plans for its bullous keratopathy stem cell therapy CSL-001.

Bio Pharma Dive
JUNE 28, 2023
The companies aim to seek an FDA nod for their bispecific antibody, Epkinly, in follicular lymphoma, where it would compete with a recently approved Roche medicine.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Rethinking Clinical Trials
JUNE 28, 2023
In this Friday’s PCT Grand Rounds, Adrian Hernandez, Pamela Tenaerts, and Craig Lipset will present “Decentralized Trials – From Guidance to Reality & What’s Left.” The Grand Rounds session will be held on Friday, June 30, 2023, at 1:00 pm eastern. Dr. Hernandez is a professor of medicine and director of the Duke Clinical Research Institute at Duke University; he is a co–principal investigator of the NIH Pragmatic Trials Collaboratory Coordinating Center.

Pharmaceutical Technology
JUNE 28, 2023
At the first South America Summit in nine years, Brazil-led plans of continental reintegration position the region’s largest healthcare market as a springboard for the pharmaceutical industry.

Bio Pharma Dive
JUNE 28, 2023
A new rule proposed by antitrust regulators would ask companies to provide additional information about planned mergers to help the Federal Trade Commission keep pace with increased deal volume and complexity.

Pharmaceutical Technology
JUNE 28, 2023
Nordic healthcare providers are leading the momentum in enhancing ESG considerations. We examine the regional developments that will provide a competitive edge to pharma manufacturers.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
JUNE 28, 2023
As well as exiting the San Diego site, Illumina is “evaluating its options” for another California campus in Foster City.
Pharmaceutical Technology
JUNE 28, 2023
Shares in Centogene rose by 41% following news of the collaboration with the Saudi government-backed biopharma company.

BioPharma Reporter
JUNE 28, 2023
Dr. Theresa Heah is the CEO of Intergalactic Therapeutics, bringing over two decades of global leadership experience in ophthalmology drug development along with commercialization in early-stage, private-staged companies. We spoke to her about her background, overcoming adversity and unlocking the power of science. Losing her father at 5 years old due to a lack of medical advancement in her native Malaysia is what, she says, drove and determined her career.
Pharmaceutical Technology
JUNE 28, 2023
The Australian TGA has granted provisional approval for Specialised Therapeutics’ Minjuvi regimen for treating diffuse large B-cell lymphoma.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharma Times
JUNE 28, 2023
Trial involved over 450 women across Europe, Turkey and Canada who were unsuitable for hormone therapy - News - PharmaTimes
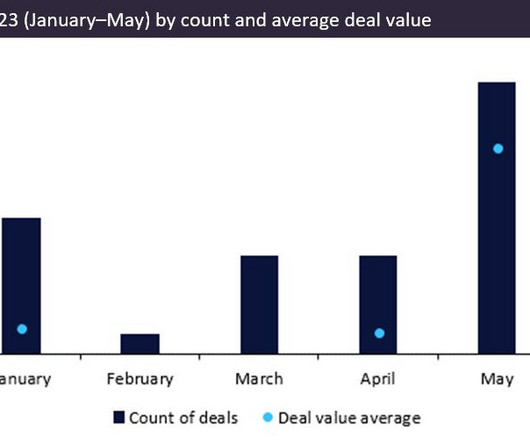
Pharmaceutical Technology
JUNE 28, 2023
Between January and May 2023, pharma CMO deals were scarce, with only 32 deals, a consequence of high inflation and interest rates.

Drug Discovery World
JUNE 28, 2023
Ernesto Staroswiecki, Senior Manager, R&D, at Beckman Coulter Life Sciences takes readers through common lab automation myths. Many clinical and research laboratories today are having to do more with less: faced with ongoing staffing shortages, increased workloads, and budgetary constraints, many laboratory managers are feeling the stress, which is where the benefits of automation can be truly realised for labs in both academia and industry.

Pharmaceutical Technology
JUNE 28, 2023
SK bioscience and the Peter Doherty Institute have signed a research collaboration deal for the development of a influenza vaccine.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

XTalks
JUNE 28, 2023
Gene therapies for Duchenne muscular dystrophy (DMD) have been an area of intense research and Sarepta’s Elevidys is now the first one to be approved by the US Food and Drug Administration (FDA). Elevidys was granted FDA accelerated approval for the treatment of pediatric patients four to five years of age with DMD who have a confirmed mutation in the DMD gene.

Pharmaceutical Technology
JUNE 28, 2023
Pfizer has announced that the FDA has approved Ngenla (somatrogon-ghla), a paediatric growth hormone deficiency treatment.

WCG Clinical
JUNE 28, 2023
Princeton, NJ, June 28, 2023 — WCG, one of the world’s leading providers of solutions that measurably improve the quality, efficiency, and safety of clinical research, recently announced a partnership with Mint Medical to leverage its mint Lesion™ radiology platform for oncology trials. The mint Lesion™ software is used for standardized and computer-assisted review of medical imaging according to defined protocols, guidelines, and workflows.

XTalks
JUNE 28, 2023
Oterra , the leading global supplier of naturally derived colors, recently unveiled innovative red and pink color blends specifically designed for plant-based meat and seafood alternatives. The company claims that its ColorFruit and FruitMax blends can closely replicate the appearance and characteristics of traditional animal-derived products. These blends incorporate a unique combination of pigments that undergo color transformation while cooking, emulating the changes typically observed in ani

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Commerce
JUNE 28, 2023
In an interview with Pharma Commerce Editor, Nicholas Saraceno, Courtney Granville, Director, Scientific Affairs, Drug Information Association, discusses issues impacting broader supply chains.

Fierce Pharma
JUNE 28, 2023
Eco-minded AstraZeneca speaks for the trees. | AstraZeneca—often on the frontlines of environmental stewardship—is expanding its AZ Forest program to the tune of $400 million. With that new investment, the company is raising its commitment to plant and nurture 200 million trees across six continents by 2030—the same year AstraZeneca aims to halve its environmental footprint across its entire value chain.

Pharma Times
JUNE 28, 2023
GLORIA trial shows increased survival levels among newly diagnosed brain cancer patients - News - PharmaTimes

BioSpace
JUNE 28, 2023
Eli Lilly announced Thursday it will acquire former collaborative partner Sigilon Therapeutics to deepen its diabetic foothold with a potentially functional cure for Type 1.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
JUNE 28, 2023
Eton Pharmaceuticals’ dehydrated alcohol injection didn’t pass muster with the FDA, instead receiving a complete response letter (CRL) raising flags the company believes are “addressable.” | The dehydrated alcohol injection was turned down due to problems with its chemistry manufacturing and controls, issues which the company believes are addressable, it said in a statement.

BioSpace
JUNE 28, 2023
The company Thursday reported positive topline results for a Phase III trial of its Alzheimer’s-related agitation treatment, while also disclosing improprieties by a principal investigator.

Fierce Pharma
JUNE 28, 2023
Merely a month into an FDA approval, AbbVie and Genmab’s bispecific drug Epkinly has chalked up a positive readout that might enable an expansion in blood cancer, although the exact regulatory path | Merely a month into an FDA approval, AbbVie and Genmab’s bispecific drug Epkinly has chalked up a positive readout that might enable an expansion in blood cancer, although the exact regulatory path remains unclear.

BioSpace
JUNE 28, 2023
After a series of milestone approvals in the first half of 2023, the FDA is slated to decide on four more firsts before the year’s end.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content