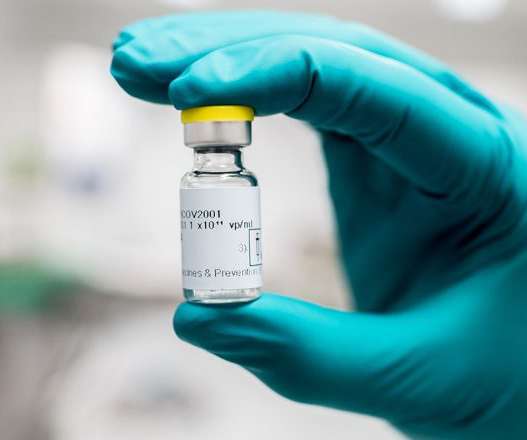
Moderna says it won't enforce coronavirus vaccine patents during pandemic
Bio Pharma Dive
OCTOBER 8, 2020
The biotech, a frontrunner in the coronavirus vaccine race, said it won't tie up resources defending the intellectual property covering its experimental shot as long as the pandemic continues.


































Let's personalize your content