Faster FDA Approvals Don’t Affect Generic Drug Availability or Cost
Pharmacy Checkers
JULY 11, 2019
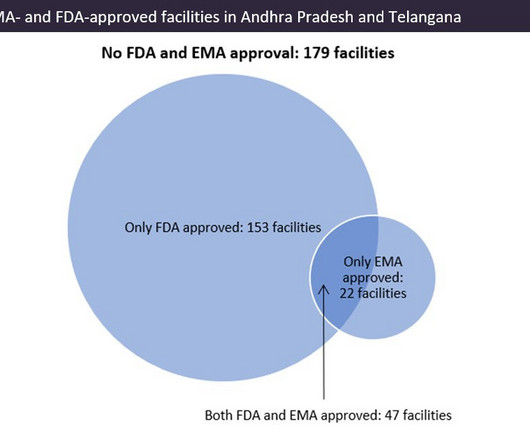
I’m proud to share that PharmacyChecker has published a white paper that examines prices and availability of newly approved generic drugs. Out of 40 generic medications that were approved from 2017 to 2018, PharmacyChecker research found the following: 42.5% (17) are not available in the U.S.































Let's personalize your content