Specialised Therapeutics’ breast cancer drug gets Philippines FDA approval
Pharmaceutical Technology
JULY 8, 2022
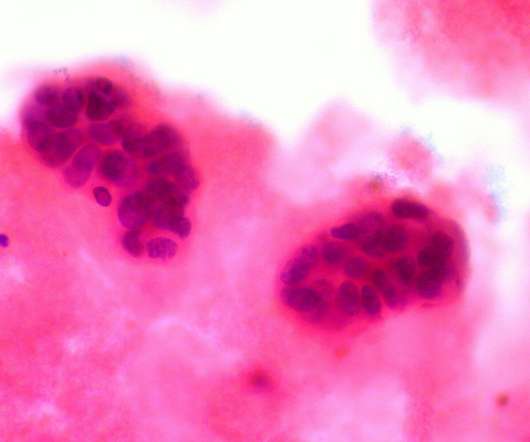
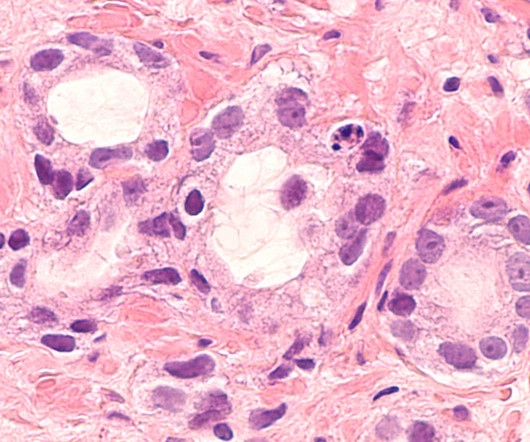
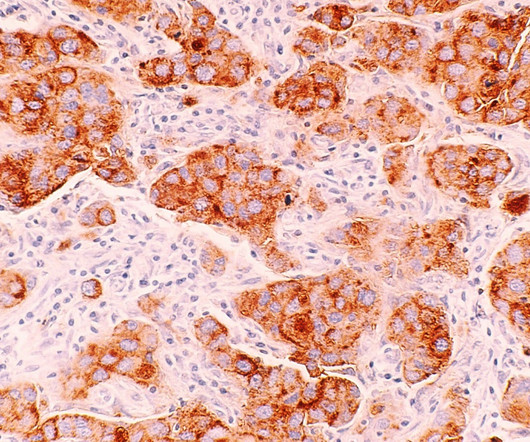
The company claimed that the utmost benefit is reported in hormone-receptor positive (HR+) women and those who commence treatment with Nerlynx within a year of receiving trastuzumab-based therapy. In a Phase III ExteNET clinical trial, neratinib offered a 34% decline in the risk of recurrence and a 2.3%



































Let's personalize your content