Cell and gene therapy companies trip at scalability hurdle
Pharmaceutical Technology
APRIL 29, 2024
Experts hold scalability challenges and high costs accountable for market failures within the cell and gene therapy landscape.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Pharmaceutical Technology
APRIL 29, 2024
Experts hold scalability challenges and high costs accountable for market failures within the cell and gene therapy landscape.

Pharmaceutical Technology
NOVEMBER 21, 2023
According to several key opinion leaders (KOLs) interviewed by GlobalData, pipeline gene therapy holds the greatest potential to transform the Gaucher disease landscape
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 5, 2023
Kyowa will pay nearly $400 million to acquire Orchard, which sells the gene therapy Libmeldy in Europe but has struggled to find paths to market for other experimental treatments.

Bio Pharma Dive
MARCH 31, 2023
The biotech tools supplier is acquiring Polyplus, a French company that makes components essential to the viral vector backbones of gene therapies.
Bio Pharma Dive
JUNE 27, 2023
The treatment could become Pfizer’s first marketed gene therapy, an area the pharma has poured significant resources into in recent years.

BioSpace
MAY 8, 2024
As Sarepta Therapeutics moves closer to full approval and an expanded label for its gene therapy, some experts push back on clinical efficacy and cost while others note the hope it provides patients with Duchenne muscular dystrophy.
Pharmaceutical Technology
FEBRUARY 10, 2023
4D Molecular Therapeutics (4DMT), the California-based biotechnology company focused on developing gene therapies for rare and large market diseases, has had the FDA place a clinical hold onto its Fabry disease (FD) gene therapy program (4D-310).
Bio Pharma Dive
MARCH 24, 2022
At least nine biotechs working in cell or gene therapy have announced layoffs, cost cuts or restructured their research since December — restructurings that have coincided with a stock market downturn.

Pharmaceutical Technology
JUNE 19, 2023
Indian pharmaceutical company Laurus Labs has signed a memorandum of agreement (MoA) with the Indian Institute of Technology, Kanpur (IIT Kanpur) to bring new gene therapy products to the market. Laurus Labs will be responsible for launching these products in India and emerging markets.

Pharmaceutical Technology
OCTOBER 25, 2022
Ast ellas Pharma has announced plans to make a strategic investment to back the development of Taysha Gene Therapies’ adeno-associated virus (AAV) development programmes for Rett syndrome and giant axonal neuropathy (GAN). Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Bio Pharma Dive
JULY 20, 2022
The Swiss drugmaker, which already owns rights to a marketed gene therapy for inherited vision loss, will work with startup Avista Therapeutics to develop better delivery tools for the complex treatments.
Bio Pharma Dive
JUNE 24, 2022
The decision brings what could be the first approved hemophilia gene therapy, Roctavian, closer to market, after a series of regulatory setbacks that have delayed its arrival.

Pharmaceutical Technology
APRIL 3, 2023
The deal will see Polyplus join the German life science group’s portfolio allowing the latter to leverage expertise in transfection reagents and plasmid DNA for gene therapy. Polyplus, based in Strasbourg, France, produces key components in the production of viral vectors used in cell and gene therapies.

BioSpace
APRIL 21, 2024
From gene-corrected cell therapies to a new CAR-T, the cell and gene therapy space looks to expand its reach into the market.

Bio Pharma Dive
MAY 21, 2021
The treatment, for a progressive, often deadly brain disease, could soon become Bluebird's third approved product and one of only a handful of marketed gene therapies in the world.
Pharmaceutical Technology
JANUARY 5, 2023
Capsida Biotherapeutics and Eli Lilly and Company ’s wholly owned subsidiary Prevail Therapeutics have announced a partnership for the development of non-invasive gene therapies for central nervous system (CNS) diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Pharmaceutical Technology
MARCH 10, 2023
On 10 March, the National Health Service Blood and Transplant (NHSBT) opened a new Clinical Biotechnology Centre (CBC) with the aim of improving the UK’s ability to develop and manufacture cell and gene therapies. The NHSBT hopes the CBC will increase the UK’s competitiveness within the market.

Bio Pharma Dive
MAY 17, 2022
The treatment, which is designed to treat Sanfilippo syndrome, could become Ultragenyx's first gene therapy to reach market in the U.S., according to the biotech's CEO.

Bio Pharma Dive
JULY 21, 2021
The approval of Skysona for a genetic brain disease is a milestone for one of gene therapy's pioneering companies, but isn't expected to turn Bluebird's financial fortunes around.
Pharmaceutical Technology
AUGUST 25, 2022
The European Commission (EC) has granted conditional marketing authorisation (CMA) for BioMarin Pharmaceutical ’s gene therapy, Roctavian (valoctocogene roxaparvovec), to treat adults with severe haemophilia A (congenital Factor VIII deficiency). Topic sponsors are not involved in the creation of editorial content.

Bio Pharma Dive
OCTOBER 21, 2021
The biotech won EU approval for two gene therapies, Zynteglo and Syksona, both of which it's now said it will pull from market after difficulties negotiating reimbursement.

Pharmaceutical Technology
SEPTEMBER 30, 2022
After several setbacks, bluebird bio bounces back with two major FDA gene therapy approvals. Last month, Zynteglo (betibeglogene autotemcel), or beti-cel, was approved as a one-time potentially curative gene therapy for patients with beta-thalassaemia who require regular blood transfusions.
Drug Discovery World
MAY 2, 2024
Hailed as a revolution in the treatment of many diseases, cell and gene therapy (CGT) is the fastest growing area of therapeutics. Download this In Focus eReport to gain a full understanding of the current market for CGTs and the areas of greatest commercial potential for the future.

Pharmaceutical Technology
NOVEMBER 9, 2022
On November 2, the Institute for Clinical and Economic Review (ICER) released its updated evidence aimed at measuring the clinical effectiveness and cost of the two haemophilia gene therapies. Known by the brand name Roctavian, BioMarin’s haemophilia A therapy valoctocogene roxaparvovec could be fairly priced in the range of $1.95–1.96
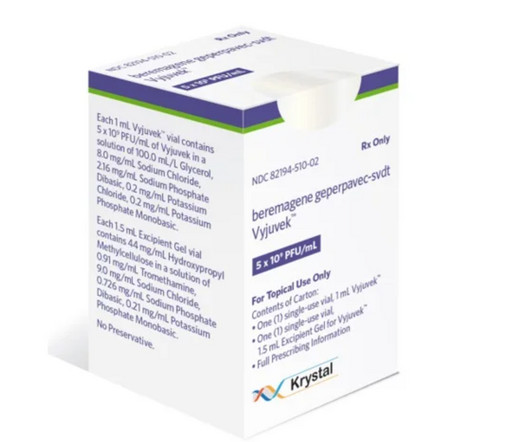
Fierce Pharma
MAY 7, 2024
After a 2023 approval from the FDA, Krystal Biotech has collected more than $95 million from its launch of Vyjuvek, the first treatment for the rare skin disease dystrophic epidermolysis bullo | The topical gene therapy has pulled in nearly $100 million during its first three quarters on the U.S.

pharmaphorum
AUGUST 10, 2021
Europe market has proved so hostile to gene therapies when it comes to pricing and reimbursement for gene therapies that bluebird bio has decided to quit the market altogether, according to Andrew Obenshain, president of its severe genetic diseases unit. market and will wind down in Europe.

Bio Pharma Dive
AUGUST 18, 2022
Its market launch is likely to be watched carefully by other gene therapy developers. Approved Wednesday for severe beta thalassemia, Zynteglo will test insurers’ willingness to pay for expensive one-time treatments.

Pharmaceutical Technology
APRIL 13, 2023
Takeda has announced that it will be pivoting away from its discovery and preclinical programmes in adeno-associated virus (AAV) gene therapies. Takeda’s announcement underlines the risk associated with gene therapy R&D at the preclinical stage and the fact that many current AAV programs are unlikely to reach late-stage trials.
Drug Discovery World
FEBRUARY 5, 2024
Mark Treherne will talk about advanced therapies and the development of RNA gene therapy for aggressive cancers which is being carried out at Spliceor. Doug Danison will discuss transitioning innovative science into commercial success in the areas of CGTs.
Pharmaceutical Technology
JULY 7, 2022
For many decades, investigators have been working on innovative therapeutic modalities known as cell and gene therapies, which use modified versions of the body’s own cellular and genetic material to treat and potentially cure these diseases. A new frontier in cancer research.
XTalks
JANUARY 4, 2024
Pfizer has kickstarted the new year with its first-ever gene therapy approval, awarded by Health Canada to the company’s Beqvez (fidanacogene elaparvovec) for the treatment of hemophilia B. There is a significant focus on developing gene therapies as longer-term solutions for the disease.

Pharmaceutical Technology
JULY 22, 2022
Pipeline therapies within the diabetic macular oedema (DME) space have recently gathered interest following the American Society of Retina Specialists (ASRS) Annual Meeting, which took place on 13–16 July.

Bio Pharma Dive
MARCH 20, 2024
million list price, the highest of any genetic medicine to come to market. Orchard is counting on the long-term data it’s accrued to convince insurers to cover Lenmeldy’s $4.25

Drug Discovery World
APRIL 30, 2024
Download this In Focus eReport to gain a full understanding of the current market for CGTs and the areas of greatest commercial potential for the future.
Drug Discovery World
FEBRUARY 13, 2024
In this webinar, hosted by DDW and sponsored by Astrea Bioseparations, you will learn about how market growth can be sustained to maximise on opportunities in cell and gene therapy (CGT). The post Cell & gene therapies: How can market growth be sustained to maximise on opportunities?
Bio Pharma Dive
OCTOBER 25, 2022
Treatment is now mostly of infants newly diagnosed with spinal muscular atrophy, as many of those previously eligible either already received Zolgensma or other drugs from Biogen and Roche.

Pharmaceutical Technology
MAY 25, 2023
Pushing back an initial deadline, the US Food and Drug Administration (FDA) has proposed a new regulatory action date of 22 June, by which time the agency will assess the logistics of a possible approval for Sarepta Therapeutics’ Duchenne muscular dystrophy (DMD) gene therapy.
Pharma Mirror
JANUARY 30, 2022
Ben Beckley, Global Lead at EmerGENE explores the market challenges holding back Cell and Gene Therapy (C>) from taking its place as an established treatment area. The global market is projected to reach $13.8 He explains how we can navigate obstacles to ensure C> can achieve its full potential.

Pharmaceutical Technology
DECEMBER 14, 2022
Merck and Synplogen have signed a non-binding Memorandum of Understanding (MoU) to expedite the development and manufacturing of viral vector-based gene therapy applications. The firms intend to merge their expertise to provide simplified viral vector gene therapy development, production and testing in Japan.

Pharmaceutical Technology
JUNE 19, 2023
Although only a small number of gene therapies have reached the market thus far, the industry is poised to grow quickly over the next few years. According to GlobalData’s clinical trials database, there are currently 1,231 planned and ongoing trials for gene therapies and gene-modified cell therapies alone.

Drug Discovery World
JUNE 23, 2023
Sarepta Therapeutics’ Elevidys has become the first gene therapy for Duchenne muscular dystrophy (DMD) to gain marketing authorisation in the US. It is contraindicated in patients with any deletion in exon 8 and/or exon 9 in the DMD gene.

Bio Pharma Dive
JULY 9, 2021
Zynteglo sales have been on hold since February, when a patient in a clinical trial of another, related Bluebird medicine developed leukemia.
pharmaphorum
DECEMBER 16, 2022
CSL’s gene therapy for haemophilia B has been recommended for approval by the EMA’s human medicine committee, setting up a decision by the European Commission early next year. The post CSL closes on EU approval for haemophilia B gene therapy appeared first on.
BioPharma Reporter
AUGUST 24, 2023
Cell and Gene Therapy Catapult, Rentschler Biopharma, and Refeyn have announced a new partnership to develop and apply âinnovativeâ process analytical technologies (PAT) to improve the process and efficiency of AAV manufacture.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content