FDA panel backs bluebird’s CALD gene therapy, despite safety worries
pharmaphorum
JUNE 10, 2022
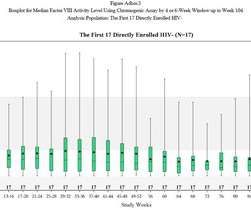
The FDA may have safety concerns abut bluebird bio’s gene therapy for rare, fatal disease cerebral adrenoleukodystrophy (CALD), but its advisors believe its benefits far outweigh the risks. bluebird is also conducting the phase 3 ALD-104 trial of eli-cel in CALD, which is due to generate results in 2024.
























Let's personalize your content