Lilly and ProQR to expand genetic medicine development agreement
Pharmaceutical Technology
DECEMBER 23, 2022
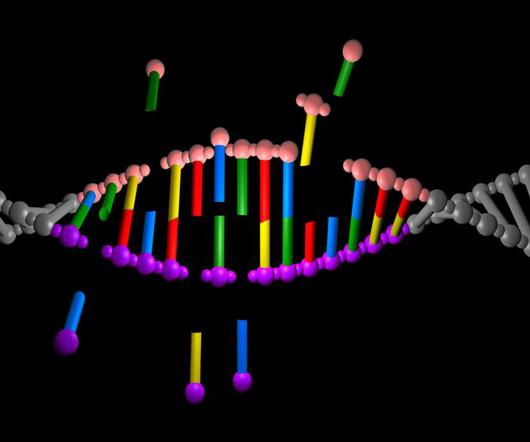
Eli Lilly and Company has expanded a licencing and partnership agreement with ProQR Therapeutics to discover, develop and market new genetic medicines. The post Lilly and ProQR to expand genetic medicine development agreement appeared first on Pharmaceutical Technology.







































Let's personalize your content