Health Canada gives approval to Enhertu for breast cancer treatment
Pharmaceutical Technology
JANUARY 13, 2023
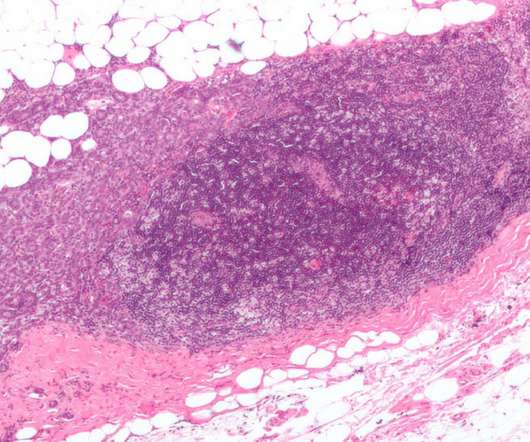
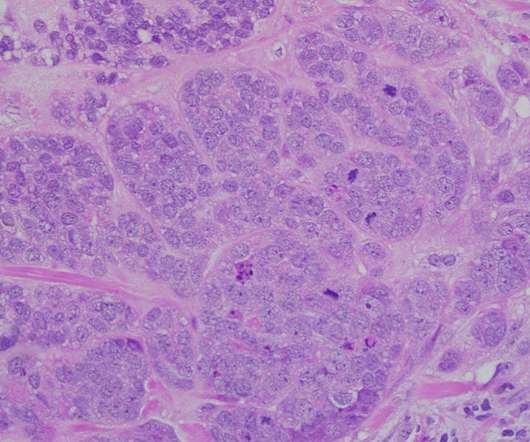
The regulatory approval was based on the data obtained from the DESTINY-Breast04 Phase III trial, which was conducted in HR-positive or HR-negative, HER2-low unresectable or metastatic breast cancer patients, who have received one or two prior lines of chemotherapy.












































Let's personalize your content