Cancer patients overlooked in COVID-19 vaccine rollout
Scienmag
DECEMBER 2, 2021
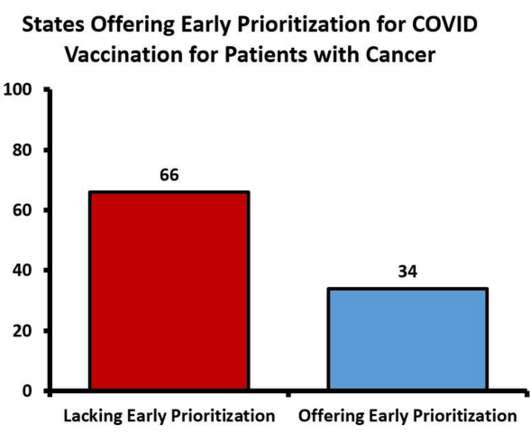
states failed to prioritize cancer patients for COVID-19 vaccinations, despite recommendations from the Centers for Disease Control and Prevention (CDC), according to a study being presented today at the annual meeting of the Radiological Society of North America (RSNA). CHICAGO – Almost two-thirds of U.S.






















Let's personalize your content