Why Biotech Sponsors Need Outside Support: IRB, IBC, DMCs and EACs
WCG Clinical
SEPTEMBER 5, 2023
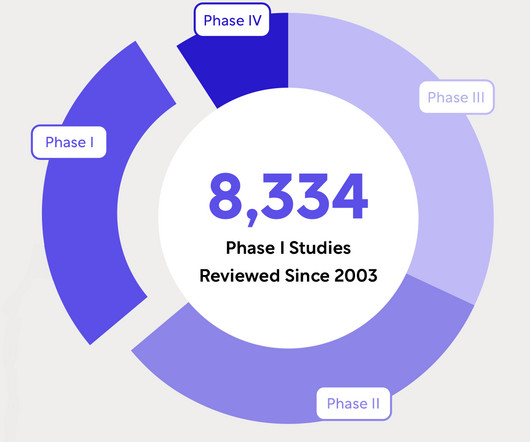
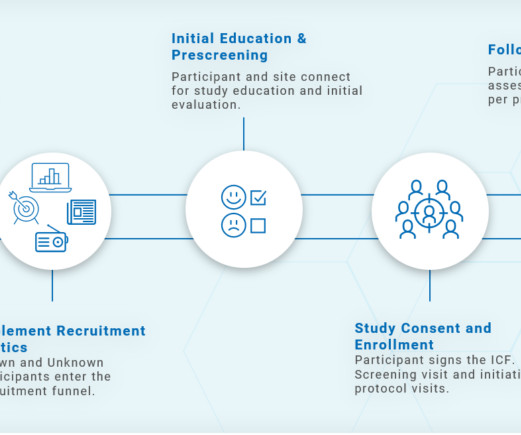
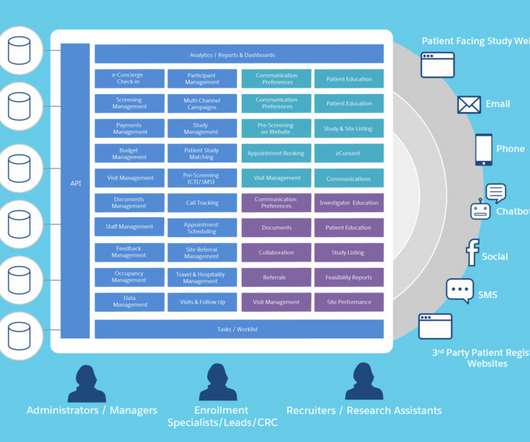
Speed, quality, predictability, and risk management are a few of the many reasons biotech sponsors need one partner for all study reviews. Assess prospective site lists. Download Your Free Copy Today The post Why Biotech Sponsors Need Outside Support: IRB, IBC, DMCs and EACs appeared first on WCG.














































Let's personalize your content