Cartherics grants licence for CTH-004 to Shunxi
Pharmaceutical Technology
MAY 1, 2023
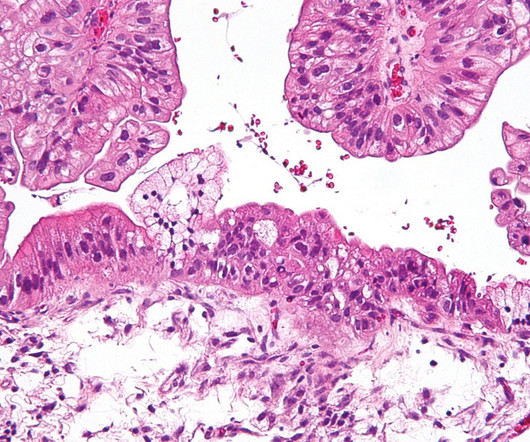
Australian biotechnology company Cartherics has granted licence for CTH-004 , its autologous CAR-T cell product, to Chinese company Shunxi. Cartherics will retain all the development and commercialisation rights for the therapy outside Greater China. We hope that women worldwide will benefit from this therapy.”


























Let's personalize your content