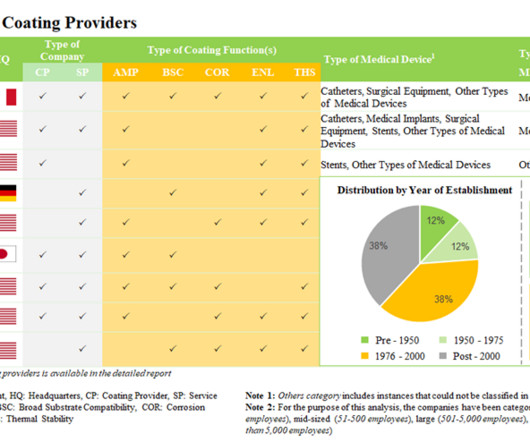
Medical Device Innovation Summit: India is poised to be a manufacturing hub for medical equipments and devices
Pharma Mirror
SEPTEMBER 4, 2020
The first digital session of Medical Device Innovation Summit concludes on a positive note with industry leaders addressing burning topics to promote innovation in the medical equipment industry.
















































Let's personalize your content