Top 5 Most Promising FDA New Drug Approvals Expected in the Second Half of 2024
XTalks
MAY 2, 2024
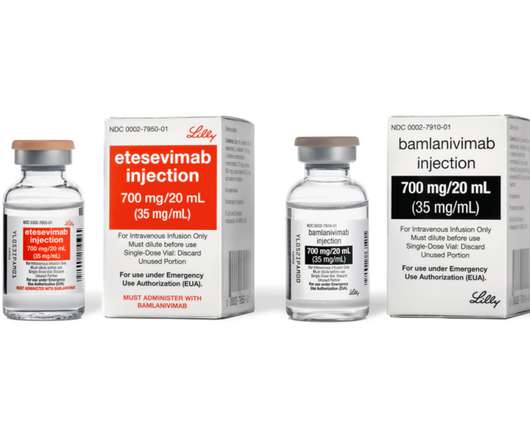
Some treatments already on the market have grabbed additional approvals in 2024, including Novo Nordisk’s Wegovy (semaglutide) for cardiovascular risk reduction, Dupixent (dupilumab) for eosinophilic esophagitis (EoE) in young children and Xolair (omalizumab) for food allergies. The condition is an adverse event linked to competitor vaccines.




































Let's personalize your content