GreenLight and US NIH partner to develop Covid-19 vaccine for variants
Pharmaceutical Technology
AUGUST 1, 2022
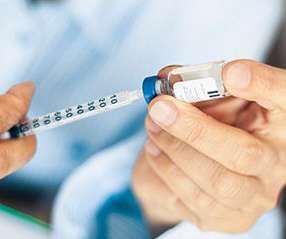
GreenLight Biosciences has entered a partnership with the US National Institutes of Health (NIH) for the development of Covid-19 vaccines, which offer broader protection against new variants and with durable effects. They intend to develop vaccines that provide lasting immune responses compared to existing vaccines.































Let's personalize your content