Covid-19 induced immune response may damage brain, NINDS study finds
Pharmaceutical Technology
JULY 6, 2022
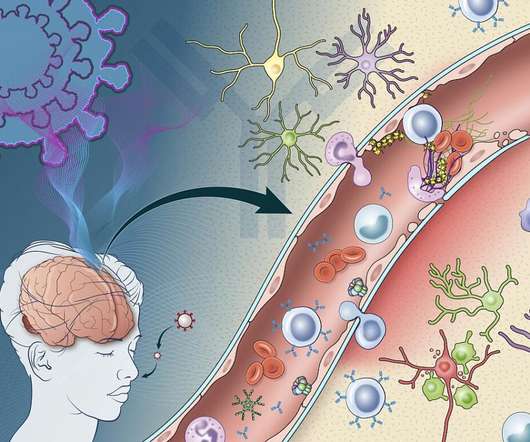
Scientists at the National Institutes of Health (NIH) unit National Institute of Neurological Disorders and Stroke (NINDS) have found that Covid-19-induced immune response could damage the blood vessels of the brain and may lead to short and long-term neurological symptoms. .



















Let's personalize your content