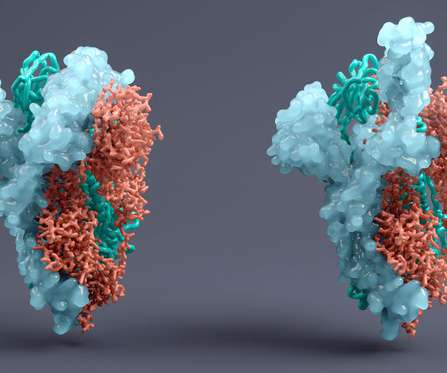
STAT+: Drugmakers ask regulators to change standards on new Covid antibody drugs for most vulnerable
STAT News
DECEMBER 15, 2022
and European regulators on Thursday to adopt new standards for approving antibody drugs against Covid, particularly for immunocompromised and other vulnerable patients. Biotech executives and a handful of academics pleaded with U.S.






































Let's personalize your content