The democratisation of cell and gene therapy
Drug Discovery World
MAY 29, 2024
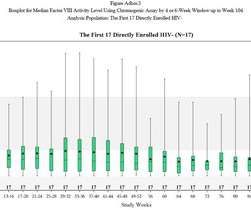
Marc Hummersone, Senior Director of Research and Development (R&D) at Astrea Bioseparations, shares insight on the challenges and opportunities in cell and gene therapy (CGT) with DDW’s Megan Thomas. So yes, you’ve got protein, but it’s not going to transfect or infect.” That’s not the case in CGT.”






































Let's personalize your content