Amgen’s Tarlatamab Gets FDA Priority Review for Small Cell Lung Cancer
XTalks
DECEMBER 29, 2023
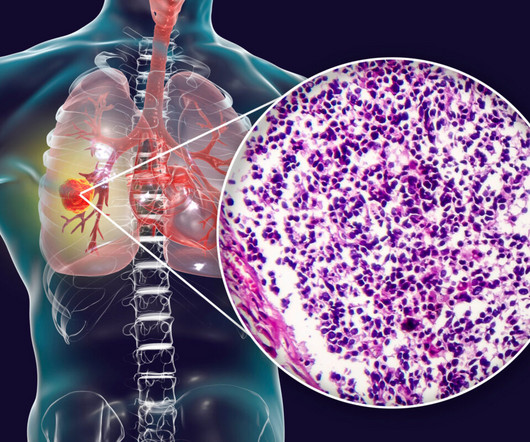
Tarlatamab is an investigational drug currently undergoing clinical trials to assess its efficacy in patients with two distinct types of neuroendocrine cancer: SCLC and neuroendocrine prostate cancer (NEPC). New Drugs Approved for Small-Cell Lung Cancer Several drugs have received FDA approval for treating SCLC.

















































Let's personalize your content